Ion transporter
In biology, an ion transporter (or ion pump) is a transmembrane protein that moves ions across a plasma membrane against their concentration gradient through active transport.[1] These primary transporters are enzymes that convert energy from various sources—including Adenosine triphosphate(ATP), sunlight, and other Redox reactions—to potential energy stored in an electrochemical gradient. This potential energy is then used by secondary transporters, including ion carriers and ion channels, to drive vital cellular processes, such as ATP synthesis.[2]
Classification and Disambiguation
Ion transporters are classified as a super family of transporters that contain 12 families of transporters.[3] These families are part of the Transport Classification (TC) system that is used by the International Union of Biochemistry and Molecular Biology (IUBMB) and are grouped according to characteristics such as the substrates being transported, the transport mechanism, the energy source used, and also by comparing the DNA sequences making up each protein. The most important unifying factor being the charged nature of the substrate which indicates the transport of an ion and not a neutral species.[3]
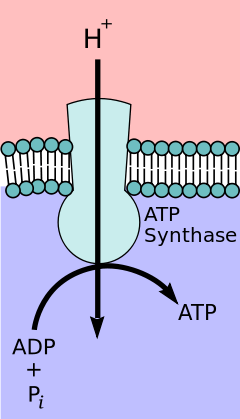
Ion transporters differ significantly from ion channels. An electrochemical gradient or concentration gradient is a difference in concentration of a chemical molecule or ion in two separate areas.[4] At equilibrium the concentrations of the ion in both areas will be equal, so if there is a difference in concentration the ions will seek to flow "down" the concentration gradient or from a high concentration to low concentration. Ion channels allows the specific ions that will fit into the channel to flow down their concentration gradient, equalizing the concentrations on either side of the cell membrane. Ion channels accomplish this via facilitated diffusion which is a type of passive transport. In contrast, ion transporters perform active transport by moving ions against their concentration gradient.[1] Using energy sources such as ATP, ion transporters are able to move ions against their concentration gradient which can then be used by secondary transporters or other proteins as a source of energy.[4]
Energy Source
Primary Transport
Primary transporters use energy to transport ions such as Na +, K+, and Ca2+ across a cells membrane and can create concentration gradients.[4] This transport usually uses ATP as an energy source but can also generate ATP through methods such as the electron transport chain in plants.[1][4]
ATP Utilizing
Transporters that use ATP convert the energy in ATP into potential energy in the form of a concentration gradient. They use the ATP to transport an ion from a low concentration to a higher concentration. Examples of proteins that use ATP are P-type ATPases that transfer Na +, K+, and Ca2+ ions by phosphorylation, A-type ATPases that transfer anions, and ABC transporters (ATP binding and cassette transporters) that transport a broad set of molecules.[4] Examples of the P-type ATPase include Na+/K+-ATPase [1] that is regulated by Janus Kinase-2[5] as well as Ca2+ ATPase which exhibits sensitivity to ADP and ATP concentrations[2] P-glycoprotein is an example of an ABC transport binding protein in the human body.
ATP Producing
ATP Producing transporters run in the opposite direction of ATP Utilizing transporters. These proteins transport ions from high to low concentration with the gradient but in the process ATP is formed. Potential energy in the form of the concentration gradient is used to generate ATP.[4] In animals, this ATP synthesis takes place in the mitochondria using F- type ATPase otherwise known as ATP synthase. V-type ATPase serves the opposite function as F-type ATPase and is used in plants to hydrolyze ATP to create a proton gradient. Examples of this are lysosomes that use V-type ATPase acidify vesicles or plant vacuoles during process of photosynthesis in the chloroplasts.[1] This process can be regulated through various methods such as pH.[6]
Secondary Transport
Secondary Transporters also transport ions against the concentration gradient - from low concentration to high concentration - but unlike primary transporters who use ATP to create a concentration gradient, secondary transporters use the potential energy from the concentration gradient created by the primary transporters to transport ions.[4] Symporters such as the Sodium-chloride symporter transport an ion with its concentration gradient, and they couple the transport of a second molecule in the same direction. Antiporters also use the concentration gradient but the coupled molecule is tranported in the opposite direction.[4]
Regulation
Ion transporters can be regulated in a variety of different ways such as phosphorylation, allosteric inhibition or activation, and sensitivity to ion concentration. Using protein kinases to add a phosphate group or phosphatases to dephosphorylate the protein can change the activity of the transporter.[7] Whether the protein is activated or inhibited with the addition of the phosphate group depends on the specific protein. With allosteric inhibition, the regulatory ligand can bind into the regulatory site and either inhibit or activate the transporter. Ion transporters can also be regulated by the concentration of an ion (not necessarily the ion it transfers) in solution. For example the electron transport chain is regulated by the precense of H+ ions (pH of the solution) in solution.[4]
Table of Ion Transporters
See also
- Wikipedia:MeSH D12.776#MeSH D12.776.157.530.450 --- ion pumps
- Ion transport number
- Transport protein
- Membrane Transport Protein
- Active Transport
External links
- Ion pumps at the US National Library of Medicine Medical Subject Headings (MeSH)
- Ion transporter superfamily
- The Transporter substrate database (TSdb)
References
- 1 2 3 4 5 T., Scheer, Bradley (2014-01-01). "Ion transport". AccessScience. doi:10.1036/1097-8542.352000.
- 1 2 Haumann, Johan (2010). "Mitochondrial Free [Ca2+] Increases during ATP/ADP Antiport and ADP Phosphorylation: Exploration of Mechanisms". Biophysical. 99: 997–1006 – via Elsevier Science Direct.
- 1 2 Prakash, Shraddha (2003). "The ion transporter superfamily". Biochimica et Biophysica Acta (BBA) - Biomembranes. 1618: 79–92 – via Elsevier Science Direct.
- 1 2 3 4 5 6 7 8 9 G.,, Voet, Judith; W.,, Pratt, Charlotte. Fundamentals of biochemistry : life at the molecular level. ISBN 9781118918401. OCLC 910538334.
- ↑ Hosseinzadeh, Zohreh (2014). "Down-Regulation of the Epithelial Na+ Channel ENaC by Janus kinase 2". The Journal of Membrane Biology. 247: 331–338.
- ↑ Tikhonov, Alexander N. (2013-05-22). "pH-Dependent regulation of electron transport and ATP synthesis in chloroplasts". Photosynthesis Research. 116 (2-3): 511–534. ISSN 0166-8595. doi:10.1007/s11120-013-9845-y.
- ↑ Marshall, William S.; Watters, Kaitlyn D.; Hovdestad, Leah R.; Cozzi, Regina R. F.; Katoh, Fumi (2009-08-01). "CFTR Cl- channel functional regulation by phosphorylation of focal adhesion kinase at tyrosine 407 in osmosensitive ion transporting mitochondria rich cells of euryhaline killifish". The Journal of Experimental Biology. 212 (Pt 15): 2365–2377. ISSN 0022-0949. PMC 2712415
 . PMID 19617429. doi:10.1242/jeb.030015.
. PMID 19617429. doi:10.1242/jeb.030015.