Ion-mobility spectrometry
Ion-mobility spectrometry (IMS) is an analytical technique used to separate and identify ionized molecules in the gas phase based on their mobility in a carrier buffer gas. Though heavily employed for military or security purposes, such as detecting drugs and explosives, the technique also has many laboratory analytical applications, including the analysis of both small and large biomolecules.[1] IMS instruments are extremely sensitive stand-alone devices, but are often coupled with mass spectrometry, gas chromatography or high-performance liquid chromatography in order to achieve a multi-dimensional separation. They come in various sizes, ranging from a few millimeters to several meters depending on the specific application, and are capable of operating under a broad range of conditions. Systems operated at higher pressure (i.e. atmospheric conditions, 1 atm or 1013 hPa) are often accompanied by elevated temperature (above 100 °C), while lower pressure systems (1-20 hPa) do not require heating.
History
IMS was first developed primarily by Earl W. McDaniel of Georgia Institute of Technology in the 1950s and 1960s when he used drift cells with low applied electric fields to study gas phase ion mobilities and reactions.[2] In the following decades, he coupled his new technique with a magnetic-sector mass spectrometer, with others also utilizing his techniques in new ways. IMS cells have since been attached to many other mass spectrometers, gas chromatographs and high-performance liquid chromatography setups. IMS is a widely used technique, and improvements and other uses are continually being developed.
Applications
Perhaps ion mobility spectrometry's greatest strength is the speed at which separations occur—typically on the order of tens of milliseconds. This feature combined with its ease of use, relatively high sensitivity, and highly compact design have allowed IMS as a commercial product to be used as a routine tool for the field detection of explosives, drugs, and chemical weapons. Major manufacturers of IMS screening devices used in airports are Morpho and Smiths Detection.
In the pharmaceutical industry IMS is used in cleaning validations, demonstrating that reaction vessels are sufficiently clean to proceed with the next batch of pharmaceutical product. IMS is much faster and more accurate than HPLC and total organic carbon methods previously used. IMS is also used for analyzing the composition of drugs produced, thereby finding a place in quality assurance and control.[3] As a research tool ion mobility is becoming more widely used in the analysis of biological materials, specifically, proteomics and metabolomics. For example, IMS-MS using MALDI as the ionization method has helped make advances in proteomics, providing faster high-resolution separations of protein pieces in analysis.[4]
Outside of laboratory purposes, IMS has found great usage as a detection tool for hazardous substances. More than 10,000 IMS devices are in use worldwide in airports, and the US Army has more than 50,000 IMS devices.[5] In industrial settings, uses of IMS include checking equipment cleanliness and detecting emission contents, such as determining the amount of hydrochloric and hydrofluoric acid in a stack gas from a process.[6] It is also applied in industrial purposes to detect harmful substances in air.[7]
In metabolomics the IMS is used to detect lung cancer, Chronic obstructive pulmonary disease, sarcoidosis, potential rejections after lung transplantation and relations to bacteria within the lung (see breath gas analysis).
Ion mobility
The physical quantity ion mobility K is defined as the proportionality factor between an ion's drift velocity vd in a gas and an electric field of strength E.
Ion mobilities are commonly reported as reduced mobilities, correcting to standard gas density n0, which can be expressed in standard temperature T0 = 273 K and standard pressure p0 = 1013 hPa. It should be noted that this does not correct for other effects than the change in gas density and the reduced ion mobility is therefore still temperature dependent.
The ion mobility K can, under a variety of assumptions, be calculated by the Mason-Schamp equation.
where Q is the ion charge, n is the drift gas number density, μ is the reduced mass of the ion and the drift gas molecules, k is Boltzmann constant, T is the drift gas temperature, and σ is the collision cross section between the ion and the drift gas molecules. Often, N is used instead of n for the drift gas number density and Ω instead σ for the ion-neutral collision cross section. This relation holds approximately at a low electric field limit, where the ratio of E/N is small and thus the thermal energy of the ions is much greater than the energy gained from the electric field between collisions. With these ions having similar energies as the buffer gas molecules, diffusion forces dominate ion motion in this case. The ratio E/N is typically given in Townsends (Td) and the transition between low- and high-field conditions is typically estimated to occur between 2 Td and 10 Td.[8] When low-field conditions no longer prevail, the ion mobility itself becomes a function of the electric field strength which is usually described empirically through the so-called alpha function.
Ionization
The molecules of the sample need to be ionized, usually by corona discharge, atmospheric pressure photoionization (APPI), electrospray ionization (ESI), or a radioactive source, e.g. a small piece of 63Ni or 241Am, similar to the one used in ionization smoke detectors. ESI and MALDI techniques are commonly used when IMS is paired with mass spectrometry.
Doping materials are sometimes added to the drift gas for ionization selectivity. For example, acetone can be added for chemical warfare agent detection, chlorinated solvents added for explosives, and nicotinamide added for drugs detection.[9]
Analyzers
Ion mobility spectrometers exist based on various principles, optimized for different applications. A review from 2014 lists eight different ion mobility spectrometry concepts.[10]
Drift tube
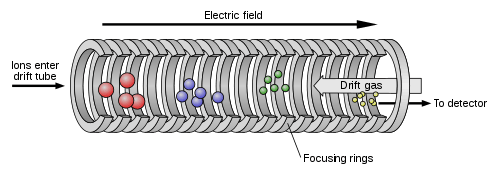
In its simplest form, an IMS system measures how long a given ion takes to traverse a given length in a uniform electric field through a given atmosphere. In specified intervals, a sample of the ions is let into the drift chamber; the gating mechanism is based on a charged electrode working in a similar way as the control grid in triodes works for electrons. For precise control of the ion pulse width admitted to the drift tube, more complex gating systems such as a Bradbury-Nielsen or a Field Switching Shutter are employed. Once in the drift tube, ions are subjected to a homogeneous electric field ranging from a few volts per centimeter up to many hundreds of volts per centimeter. This electric field then drives the ions through the drift tube where they interact with the neutral drift molecules contained within the system and separate based on the ion mobility, arriving at the detector for measurement. Ions are recorded at the detector in order from the fastest to the slowest, generating a response signal characteristic for the chemical composition of the measured sample.
The ion mobility K can then be experimentally determined from the drift time tD of an ion traversing within a homogeneous electric field the potential difference U in the drift length L.
A drift tube’s resolving power RP can, when diffusion is assumed as the sole contributer to peak broadening, be calculated as
where tD is the ion drift time, ΔtD is the Full width at half maximum, L is the tube length, E is the electric field strength, Q is the ion charge, k is Boltzmann’s constant, and T is the drift gas temperature. Ambient pressure methods allow for higher resolving power and greater separation selectivity due to a higher rate of ion-molecule interactions and is typically used for stand-alone devices, as well as for detectors for gas, liquid, and supercriticial fluid chromatography. As shown above, the resolving power depends on the total voltage drop the ion traverses. Using a drift voltage of 25 kV in a 15 cm long atmospheric pressure drift tube, a resolving power above 250 is achievable even for small, single charged ions.[11] This is sufficient to achieve separation of some isotopologues based on their difference in reduced mass μ.[12]
Low pressure drift tube
Reduced pressure drift tubes operate using the same principles as their atmospheric pressure counterparts, but at drift gas pressure of only a few torr. Due to the vastly reduced number of ion-neutral interactions, much longer drift tubes or much faster ion shutters are necessary to achieve the same resolving power. However, the reduced pressure operation offers several advantages. First, it eases interfacing the IMS with mass spectrometry.[2] Second, at lower pressures, ions can be stored for injection from an ion trap[13] and re-focussed radially during and after the separation. Third, high values of E/N can be achieved, allowing for direct measurement of K(E/N) over a wide range.[14]
Travelling wave
Though drift electric fields are normally uniform, non-uniform drift fields can also be used. One example is the travelling wave IMS,[15] which is a low pressure drift tube IMS where the electric field is only applied in a small region of the drift tube. This region then moves along the drift tube, creating a wave pushing the ions towards the detector, removing the need for a high total drift voltage. An especially noteworthy variant is the "SUPER" IMS,[16] which combines ion trapping by the so-called structures for lossless ion manipulations (SLIM) with several passes through the same drift region to achieve extremely high resolving powers.
Trapped ion mobility spectrometry
In trapped ion mobility spectrometry (TIMS), ions are held stationary (or trapped) in a flowing buffer gas by an axial electric field gradient (EFG) profile while the application of radio frequency (rf) potentials results in trapping in the radial dimension.[17] TIMS operates in the pressure range of 2 to 5 hPa and replaces the ion funnel found in the source region of modern mass spectrometers. It can be coupled with nearly any mass analyzer through either the standard mode of operation for beam-type instruments or selective accumulation mode (SA-TIMS) when used with trapping mass spectrometry (MS) instruments.
Effectively, the drift cell is prolonged by the ion motion created through the gas flow.[18] Thus, TIMS devices do neither require large size nor high voltage in order to achieve high resolution, for instance achieving over 250 resolving power from a 4.7 cm device through the use of extended separation times.[19] However, the resolving power strongly depends on the ion mobility and decreases for more mobile ions. In addition, TIMS can be capable of higher sensitivity than other ion mobility systems because no grids or shutters exist in the ion path, improving ion transmission both during ion mobility experiments and while operating in a transparent MS only mode.
High-field asymmetric waveform ion mobility spectrometry
DMS (differential mobility spectrometer) or FAIMS (field asymmetric ion mobility spectrometer) make use of the dependence of the ion mobility K on the electric field strength E at high electric fields. Ions are transported through the device by the drift gas flow and subjected to different field strengths in orthogonal direction for different amounts of time. Ions are deflected towards the walls of the analyzer based on the change of their mobility. Thereby only ions with a certain mobility dependence can pass the thus created filter
Differential mobility analyzer

DMA differential mobility analyzer make use of a fast gas stream perpendicular to the electric field. Thereby ions of different mobilities undergo different trajectories. This type of IMS corresponds to the sector instruments in mass spectrometry. They also work as a scannable filter. Examples include the DMD (Differential Mobility Detector), first commercialized in Varian CP-4900 MicroGC. Aspiration IMS operates with open-loop circulation of sampled air. Sample flow is passed via ionization chamber and then enters to measurement area where the ions are deflected into one or more measuring electrodes by perpendicular electric field which can be either static or varying. The output of the sensor is characteristic of the ion mobility distribution and can be used for detection and identification purposes.
Drift gas
The drift gas composition is an important parameter for the IMS instrument design and resolution. Often, different drift gas compositions can allow for the separation of otherwise overlapping peaks.[20] Elevated gas temperature assists in removing ion clusters that may distort experimental measurements.[21][22]
Detector
Often the detector is a simple Faraday plate coupled to a transimpedance amplifier, however, more advanced ion mobility instruments are coupled with mass spectrometers in order to obtain both size and mass information simultaneously. It is noteworthy that the detector influences the optimum operating conditions for the ion mobility experiment.[23]
Combined methods
IMS can be combined with other separation techniques.
Gas chromatography
When IMS is coupled with gas chromatography, common sample introduction is with the GC capillary column directly connected to the IMS setup, with molecules ionized as they elute from GC.[9] A similar technique is commonly used for HPLC. A novel design for corona discharge ionization ion mobility spectrometry (CD–IMS) as a detector after capillary gas chromatography has been produced in 2012. In this design, a hollow needle was used for corona discharge creation and the effluent was entered into the ionization region on the upstream side of the corona source. In addition to the practical conveniences in coupling the capillary to IMS cell, this direct axial interfacing helps us to achieve a more efficient ionization, resulting in higher sensitivity.
Liquid chromatography
Coupled with LC and MS, IMS has become widely used to analyze biomolecules, a practice heavily developed by David E. Clemmer, now at Indiana University (Bloomington).[24]
Mass spectrometry
When IMS is used with mass spectrometry, ion mobility spectrometry-mass spectrometry offers many advantages, including better signal to noise, isomer separation, and charge state identification.[2][25] IMS has commonly been attached to several mass spec analyzers, including quadropole, time-of-flight, and Fourier transform cyclotron resonance.
Dedicated software
Ion mobility mass spectrometry is a rather recently popularized gas phase ion analysis technique. As such there is not a large software offering to display and analyze ion mobility mass spectrometric data, apart from the software packages that are shipped along with the instruments. This is a small list of software that is Free Software according to the OpenSourceInitiative definition.
Proteowizard OpenMs msXpertSuite
See also
References
- ↑ Lanucara, F., Holman, S.W., Gray, C.J., and Eyers, C.E. (2014) The power of ion mobility-mass spectrometry for structural characterization and the study of conformational dynamics. Nature Chemistry 6:281-294.
- 1 2 3 Kanu AB, Dwivedi P, Tam M, Matz L, Hill HH (January 2008). "Ion mobility-mass spectrometry". J Mass Spectrom. 43 (1): 1–22. PMID 18200615. doi:10.1002/jms.1383.
- ↑ O'Donnell, Ryan M.; Sun, Xiaobo; Harrington, Peter (2008). "Pharmaceutical applications of ion mobility spectrometry". Trends in Analytical Chemistry. 27 (1): 44–53. doi:10.1016/j.trac.2007.10.014.
- ↑ McLean, J.A.; et al. (2005). "Ion mobility–mass spectrometry: a new paradigm for proteomics". International Journal of Mass Spectrometry. 240: 301–315. doi:10.1016/j.ijms.2004.10.003.
- ↑ Zolotov, Yu. A. (2006). "Ion Mobility Spectrometry". Journal of Analytical Chemistry. 61 (6): 519. doi:10.1134/s1061934806060013.
- ↑ Particle Measuring Systems, Inc. “Ion Mobility Spectrometry (IMS) Theory and Applications”
- ↑ Räsänen, Riikka-Marjaana; Nousiainen, Marjaana; Peräkorpi, Kaleva; Sillanpää, Mika; Polari, Lauri; Anttalainen, Osmo; Utriainen, Mikko (2008). "Determination of gas phase triacetone triperoxide with aspiration ion mobility spectrometry and gas chromatography–mass spectrometry". Analytica Chimica Acta. 623 (1): 59–65. doi:10.1016/j.aca.2008.05.076.
- ↑ "Interaction potentials and transport properties of coinage metal cations in rare gases". The Journal of Chemical Physics. 127 (15): 154309. 2007-10-16. ISSN 0021-9606. doi:10.1063/1.2774977.
- 1 2 Creaser, Colin; Thomas, Paul; et al. (2004). "Ion mobility spectrometry: a review. Part 1. Structural analysis by mobility measurement". The Analyst. 129: 984–994. doi:10.1039/b404531a.
- ↑ Cumeras, R.; Figueras, E.; Davis, C. E.; Baumbach, J. I.; Gràcia, I. (2015-02-16). "Review on Ion Mobility Spectrometry. Part 1: current instrumentation". The Analyst. 140 (5): 1376–1390. ISSN 1364-5528. PMC 4331213
 . PMID 25465076. doi:10.1039/c4an01100g.
. PMID 25465076. doi:10.1039/c4an01100g. - ↑ Kirk, Ansgar T.; Zimmermann, Stefan (2015-02-21). "Pushing a compact 15 cm long ultra-high resolution drift tube ion mobility spectrometer with R = 250 to R = 425 using peak deconvolution". International Journal for Ion Mobility Spectrometry. 18 (1-2): 17–22. ISSN 1435-6163. doi:10.1007/s12127-015-0166-z.
- ↑ Kirk, Ansgar T.; Raddatz, Christian-Robert; Zimmermann, Stefan (2016-12-20). "Separation of Isotopologues in Ultra-High-Resolution Ion Mobility Spectrometry". Analytical Chemistry. ISSN 0003-2700. doi:10.1021/acs.analchem.6b03300.
- ↑ Clowers, Brian H.; Ibrahim, Yehia M.; Prior, David C.; Danielson, William F.; Belov, Mikhail E.; Smith, Richard D. (2008-02-01). "Enhanced Ion Utilization Efficiency Using an Electrodynamic Ion Funnel Trap as an Injection Mechanism for Ion Mobility Spectrometry". Analytical Chemistry. 80 (3): 612–623. ISSN 0003-2700. PMC 2516354
 . PMID 18166021. doi:10.1021/ac701648p.
. PMID 18166021. doi:10.1021/ac701648p. - ↑ Langejuergen, Jens; Allers, Maria; Oermann, Jens; Kirk, Ansgar; Zimmermann, Stefan (2014-07-15). "High Kinetic Energy Ion Mobility Spectrometer: Quantitative Analysis of Gas Mixtures with Ion Mobility Spectrometry". Analytical Chemistry. 86 (14): 7023–7032. ISSN 0003-2700. doi:10.1021/ac5011662.
- ↑ Giles, Kevin; Pringle, Steven D.; Worthington, Kenneth R.; Little, David; Wildgoose, Jason L.; Bateman, Robert H. (2004-10-30). "Applications of a travelling wave-based radio-frequency-only stacked ring ion guide". Rapid Communications in Mass Spectrometry. 18 (20): 2401–2414. ISSN 1097-0231. doi:10.1002/rcm.1641.
- ↑ Deng, Liulin; Webb, Ian K.; Garimella, Sandilya V. B.; Hamid, Ahmed M.; Zheng, Xueyun; Norheim, Randolph V.; Prost, Spencer A.; Anderson, Gordon A.; Sandoval, Jeremy A.; Baker, Erin S.; Ibrahim, Yehia M.; Smith, Richard D. (5 April 2017). "Serpentine Ultralong Path with Extended Routing (SUPER) High Resolution Traveling Wave Ion Mobility-MS using Structures for Lossless Ion Manipulations". Analytical Chemistry. 89 (8): 4628–4634. doi:10.1021/acs.analchem.7b00185.
- ↑
- M. A. Park, Apparatus and Method for Parallel Flow Ion Mobility Spectrometry Combined with Mass Spectrometry, USPN 8,288,717
- ↑ Michelmann, Karsten; Silveira, Joshua A.; Ridgeway, Mark E.; Park, Melvin A. (2014-10-21). "Fundamentals of Trapped Ion Mobility Spectrometry". Journal of The American Society for Mass Spectrometry. 26 (1): 14–24. ISSN 1044-0305. doi:10.1007/s13361-014-0999-4.
- ↑ Silveira, Joshua A.; Ridgeway, Mark E.; Park, Melvin A. (2014). "High Resolution Trapped Ion Mobility Spectrometry of Peptides". Analytical Chemistry. 86: 140528090048009. ISSN 0003-2700. PMID 24862843. doi:10.1021/ac501261h.
- ↑ Asbury, G. Reid; Hill, Herbert H. (2000-02-01). "Using Different Drift Gases To Change Separation Factors (α) in Ion Mobility Spectrometry". Analytical Chemistry. 72 (3): 580–584. ISSN 0003-2700. doi:10.1021/ac9908952.
- ↑ Bengt Nolting, Methods in Modern Biophysics, Springer Verlag, 2005, ISBN 3-540-27703-X
- ↑ Gary Eiceman & Zeev Karpas, Ion Mobility Spectrometry, CRC Press, 2005, ISBN 0-8493-2247-2
- ↑ Kirk, Ansgar T.; Allers, Maria; Cochems, Philipp; Langejuergen, Jens; Zimmermann, Stefan (2013-08-12). "A compact high resolution ion mobility spectrometer for fast trace gas analysis". The Analyst. 138 (18). ISSN 1364-5528. doi:10.1039/c3an00231d.
- ↑ Clemmer, David E.; et al. (2008). "Biomolecule Analysis by Ion Mobility Spectrometry". Annual Review of Analytical Chemistry. 1: 293–397.
- ↑ Fenn LS, McLean JA (June 2008). "Biomolecular structural separations by ion mobility-mass spectrometry". Anal Bioanal Chem. 391 (3): 905–9. PMID 18320175. doi:10.1007/s00216-008-1951-x.
Bibliography
- G.A. Eiceman; Z. Karpas (2005). Ion Mobility Spectrometry (2nd ed.). Boca Raton, FL., USA: CRC Press. ISBN 9780849322471.
- Alexandre A. Shvartsburg (2008). Differential Ion Mobility Spectrometry: Nonlinear Ion Transport and Fundamentals of FAIMS (2nd ed.). Boca Raton, FL., USA: CRC Press. ISBN 9781420051063.
- Charles L. Wilkins; Sarah Trimpin, eds. (2010). Ion Mobility Spectrometry - Mass Spectrometry: Theory and Applications. Boca Raton, FL., USA: CRC Press. ISBN 9781439813249.
- Stach, Joachim; Baumbach, Jörg I. (2002). "Ion Mobility Spectrometry - Basic Elements and Applications". International Journal for Ion Mobility Spectrometry. 5 (1): 1–21.
External links
Listing of analytical instrument sites at DMOZ