Lactam
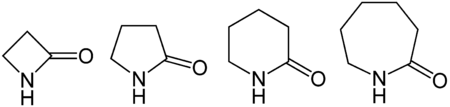
From left to right, general structures of a β-lactam, a γ-lactam, a δ-lactam, and an ε-lactam. The specific structures are β-propiolactam, γ-butyrolactam, δ-valerolactam, and ε-caprolactam.
A lactam is a cyclic amide. The term is a portmanteau of the words lactone + amide.
Nomenclature
Greek prefixes in alphabetical order indicate ring size:
- α-Lactam (3 ring atoms)
- β-Lactam (4 ring atoms)
- γ-Lactam (5 ring atoms)
- δ-Lactam (6 ring atoms)
- ε-Lactam (7 ring atoms)
This ring-size nomenclature stems from the fact that a hydrolyzed α-Lactam leads to an α-amino acid and a β-Lactam to a β-amino acid, etc.
Synthesis
General synthetic methods exist for the organic synthesis of lactams.
- Lactams form by the acid-catalyzed rearrangement of oximes in the Beckmann rearrangement.
- Lactams form from cyclic ketones and hydrazoic acid in the Schmidt reaction.
- Lactams form from cyclisation of amino acids.
- Lactams form from intramolecular attack of linear acyl derivatives from the nucleophilic abstraction reaction.
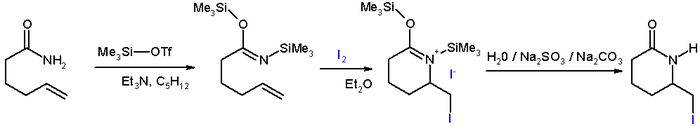
- In iodolactamization [1] an iminium ion reacts with an halonium ion formed in situ by reaction of an alkene with iodine.
- Lactams form by copper-catalyzed 1,3-dipolar cycloaddition of alkynes and nitrones in the Kinugasa reaction
- Diels-Alder reaction between cyclopentadiene and chlorosulfonyl isocyanate (CSI) can be utilized to obtain both β- as well as γ-lactam. At lower temp (−78°C), β-lactam is the preferred product. At optimum temperatures, a highly useful γ-lactam known as Vince Lactam[2] is obtained.[3]
Tautomerization to lactims
A lactim is a cyclic carboximidic acid compound characterized by an endocyclic carbon-nitrogen double bond. They are formed when lactams undergo tautomerization.
Reactions
- Lactams can polymerize to polyamides.
See also
- Lactone, a cyclic ester.
- β-Lactam
- β-Lactam antibiotics, which includes penicillins
- 2-Pyrrolidone
- 2-Piperidinone
- Caprolactam
References
- ↑ Spencer Knapp, Frank S. Gibson Organic Syntheses, Coll. Vol. 9, p.516 (1998); Vol. 70, p.101 (1992) Online article
- ↑ Singh, R.; Vince, R. Chem. Rev. 2012, 112 (8), pp 4642–4686."2-Azabicyclo[2.2.1]hept-5-en-3-one: Chemical Profile of a Versatile Synthetic Building Block and its Impact on the Development of Therapeutics"
- ↑ Pham, P.-T.; Vince, R. Phosphorus, Sulphur and Silicon 2007, 779-791.
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.