Intrastromal corneal ring segment
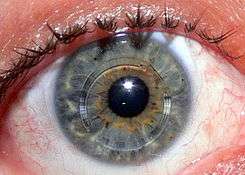
An intrastromal corneal ring segment (ICRS) (also known as intrastromal corneal ring, corneal implant or corneal insert) is a small device implanted in the eye to correct vision. The procedure involves an ophthalmologist who makes a small incision in the cornea of the eye and inserts two crescent or semi-circular shaped ring segments between the layers of the corneal stroma, one on each side of the pupil.[1] The embedding of the two rings in the cornea is intended to flatten the cornea and change the refraction of light passing through the cornea on its way into the eye.
Design
Intrastromal corneal ring segments have many different types and designs, including Intacs (US), Keraring (Brazil), Ferrara ring (Brazil),[2] and Intraseg.
Medical uses
Intrastromal corneal rings were originally used to treat mild myopia.[1] For this purpose, they have largely been superseded by excimer lasers, which have better accuracy.[1] They are now mostly used to treat mild to moderate keratoconus.[1] Intrastromal corneal rings were approved in 2004 by the Food and Drug Administration for people with keratoconus who cannot adequately correct their vision with glasses or contact lenses, and for whom corneal transplant is the only other option.[3] They were approved under the Humanitarian Device Exemption,[2][4] which means the manufacturer did not have to demonstrate effectiveness. According to the FDA, these products should not be used by people who "can achieve functional vision on a daily basis using contact lenses."[3]
References
- 1 2 3 4 Rabinowitz YS (2013). "INTACS for keratoconus and ectasia after LASIK". Int Ophthalmol Clin. 53 (1): 27–39. PMC 3653443
 . PMID 23221883. doi:10.1097/IIO.0b013e3182774453.
. PMID 23221883. doi:10.1097/IIO.0b013e3182774453. - 1 2 Zadnik K, Lindsley K (2014). "Intrastromal corneal ring segments for treating keratoconus (Protocol)". Cochrane Database of Systematic Reviews. doi:10.1002/14651858.CD011150.
- 1 2 Food and Drug Administration (26 July 2004). "INTACS Prescription Inserts for Keratoconus - H040002".
- ↑ Food and Drug Administration (9 June 2006). "Humanitarian Device Exemption (HDE)".
External links
- Intrastromal corneal ring segment (ICRS) on EyeWiki