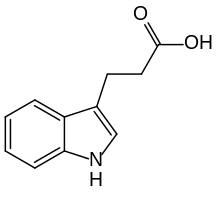
3-Indolepropionic acid
 | |
| Clinical data | |
|---|---|
| Trade names | Oxigon[1] |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| Synonyms |
Conjugate base: Indole-3-propionate |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ECHA InfoCard | 100.011.455 |
| Chemical and physical data | |
| Formula | C11H11NO2 |
| Molar mass | 189.21054 g/mol |
| 3D model (JSmol) | |
| Melting point | 134 to 135 °C (273 to 275 °F) [2] |
| |
| |
| (verify) | |
3-Indolepropionic acid (IPA), or indole-3-propionic acid, is a potent neuroprotective antioxidant and plant auxin that is being studied for therapeutic use in Alzheimer's disease.[1][2][3][4] It is endogenously produced by human microbiota and has only been detected in vivo when the species Clostridium sporogenes is present in the gastrointestinal tract.[3][4][5] As of April 2016, C. sporogenes, which uses tryptophan to synthesize indole and subsequently IPA, is the only species of bacteria known to synthesize IPA in vivo at levels which are subsequently detectable in the blood plasma of the host.[3][4][5][6]
The neuroprotective, antioxidant and anti-amyloid properties of IPA were first reported by a group of investigators in July 1999, led by Dr Pappolla and Dr. Poeggeler at the University of South Alabama.[7][8][9][10]
IPA is an even more potent scavenger of hydroxyl radicals than melatonin, the most potent scavenger of hydroxyl radicals that is synthesized by human enzymes.[2][8] Similar to melatonin but unlike other antioxidants, it scavenges radicals without subsequently generating reactive and pro-oxidant intermediate compounds.[7][2][8][11]
Tryptophan metabolism by human gastrointestinal microbiota ()
This diagram shows the biosynthesis of bioactive compounds (indole and certain derivatives) from tryptophan by bacteria in the gut.[4] Indole is produced from tryptophan by bacteria that express tryptophanase.[4] Clostridium sporogenes metabolizes indole into 3-indolepropionic acid (IPA),[3] a highly potent neuroprotective antioxidant that scavenges hydroxyl radicals.[4][2][8] In the intestine, IPA binds to pregnane X receptors (PXR) in intestinal cells, thereby facilitating mucosal homeostasis and barrier function.[4] Following absorption from the intestine and distribution to the brain, IPA confers a neuroprotective effect against cerebral ischemia and Alzheimer’s disease.[4] Lactobacillus species metabolize tryptophan into indole-3-aldehyde (I3A) which acts on the aryl hydrocarbon receptor (AhR) in intestinal immune cells, in turn increasing interleukin-22 (IL-22) production.[4] Indole itself acts as a glucagon-like peptide-1 (GLP-1) secretagogue in intestinal L cells and as a ligand for AhR.[4] Indole can also be metabolized by the liver into indoxyl sulfate, a compound that is toxic in high concentrations and associated with vascular disease and renal dysfunction.[4] AST-120 (activated charcoal), an intestinal sorbent that is taken by mouth, adsorbs indole, in turn decreasing the concentration of indoxyl sulfate in blood plasma.[4]
|
See also
References
- 1 2 Bendheim PE, Poeggeler B, Neria E, Ziv V, Pappolla MA, Chain DG (October 2002). "Development of indole-3-propionic acid (OXIGON) for Alzheimer's disease". J. Mol. Neurosci. 19 (1-2): 213–217. PMID 12212784. doi:10.1007/s12031-002-0036-0.
The accumulation of amyloid-beta and concomitant oxidative stress are major pathogenic events in Alzheimer's disease. Indole-3-propionic acid (IPA, OXIGON) is a potent anti-oxidant devoid of pro-oxidant activity. IPA has been demonstrated to be an inhibitor of beta-amyloid fibril formation and to be a potent neuroprotectant against a variety of oxidotoxins. This review will summarize the known properties of IPA and outline the rationale behind its selection as a potential disease-modifying therapy for Alzheimer's disease.
- 1 2 3 4 5 6 "3-Indolepropionic acid". Human Metabolome Database. University of Alberta. Retrieved 12 October 2015.
Indole-3-propionate (IPA), a deamination product of tryptophan formed by symbiotic bacteria in the gastrointestinal tract of mammals and birds. 3-Indolepropionic acid has been shown to prevent oxidative stress and death of primary neurons and neuroblastoma cells exposed to the amyloid beta-protein in the form of amyloid fibrils, one of the most prominent neuropathologic features of Alzheimer's disease. 3-Indolepropionic acid also shows a strong level of neuroprotection in two other paradigms of oxidative stress. (PMID 10419516 )
Origin: • Endogenous • Microbial - 1 2 3 4 Wikoff WR, Anfora AT, Liu J, Schultz PG, Lesley SA, Peters EC, Siuzdak G (March 2009). "Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites". Proc. Natl. Acad. Sci. U.S.A. 106 (10): 3698–3703. PMC 2656143
 . PMID 19234110. doi:10.1073/pnas.0812874106.
. PMID 19234110. doi:10.1073/pnas.0812874106. Production of IPA was shown to be completely dependent on the presence of gut microflora and could be established by colonization with the bacterium Clostridium sporogenes. ... Conversely, a different set of enteric bacteria has been implicated in the metabolic transformation of indole to indole-3-propionic acid (IPA) (27). IPA, also identified only in the plasma of conv mice, has been shown to be a powerful antioxidant (28) ... Although the presence of IPA in mammals has long been ascribed in the literature to bacterial metabolic processes, this conclusion was based on either the production of IPA in ex vivo cultures of individual bacterial species (31) or observed decreases in IPA levels in animals after administration of antibiotics (32). In our own survey of IPA production by representative members of the intestinal flora, only Clostridium sporogenes was found to produce IPA in culture (Table S2). Based on these results, individual GF mice were intentionally colonized with C. sporogenes strain ATCC 15579, and blood samples were taken at several intervals after colonization. IPA was undetectable in the samples taken shortly after introduction of the microbes, and was first observed in the serum 5 days after colonization, reaching plateau values comparable with conv mice by day 10. These colonization studies demonstrate that the introduction of enteric bacteria capable of IPA production in vivo into the gastrointestinal tract is sufficient to introduce IPA into the bloodstream of the host. Also, other GF animals were injected i.p. with either IPA (at 10, 20, or 40 mg/kg) or sterile PBS vehicle, and their serum concentrations of IPA were measured over time. As seen in Table S3, the high serum levels of IPA observed 1 h after injection decreased more than 90% within 5 h, showing that IPA is rapidly cleared from the blood, and that its presence in the serum of conv animals must result from continuous production from 1 or more bacterial species associated with the mammalian gut.
IPA metabolism diagram - 1 2 3 4 5 6 7 8 9 10 11 12 Zhang LS, Davies SS (April 2016). "Microbial metabolism of dietary components to bioactive metabolites: opportunities for new therapeutic interventions". Genome Med. 8 (1): 46. PMC 4840492
 . PMID 27102537. doi:10.1186/s13073-016-0296-x.
. PMID 27102537. doi:10.1186/s13073-016-0296-x. Lactobacillus spp. convert tryptophan to indole-3-aldehyde (I3A) through unidentified enzymes [125]. Clostridium sporogenes convert tryptophan to IPA [6], likely via a tryptophan deaminase. ... IPA also potently scavenges hydroxyl radicals
Table 2: Microbial metabolites: their synthesis, mechanisms of action, and effects on health and disease
Figure 1: Molecular mechanisms of action of indole and its metabolites on host physiology and disease - 1 2 Attwood G, Li D, Pacheco D, Tavendale M (June 2006). "Production of indolic compounds by rumen bacteria isolated from grazing ruminants". J. Appl. Microbiol. 100 (6): 1261–1271. PMID 16696673. doi:10.1111/j.1365-2672.2006.02896.x.
- ↑ Chyan YJ, Poeggeler B, Omar RA, Chain DG, Frangione B, Ghiso J, Pappolla MA. Potent neuroprotective properties against the Alzheimer beta-amyloid by an endogenous melatonin-related indole structure, indole-3-propionic acid. J Biol Chem. 1999 Jul 30;274(31):21937-42
- 1 2 Poeggeler B, Sambamurti K, Siedlak SL, Perry G, Smith MA, Pappolla MA. A novel endogenous indole protects rodent mitochondria and extends rotifer lifespan. PLoS One. 2010 Apr 21;5(4)
- 1 2 3 4 Chyan YJ, Poeggeler B, Omar RA, Chain DG, Frangione B, Ghiso J, Pappolla MA (July 1999). "Potent neuroprotective properties against the Alzheimer beta-amyloid by an endogenous melatonin-related indole structure, indole-3-propionic acid". J. Biol. Chem. 274 (31): 21937–21942. PMID 10419516. doi:10.1074/jbc.274.31.21937.
[Indole-3-propionic acid (IPA)] has previously been identified in the plasma and cerebrospinal fluid of humans, but its functions are not known. ... In kinetic competition experiments using free radical-trapping agents, the capacity of IPA to scavenge hydroxyl radicals exceeded that of melatonin, an indoleamine considered to be the most potent naturally occurring scavenger of free radicals. In contrast with other antioxidants, IPA was not converted to reactive intermediates with pro-oxidant activity.
- ↑ Karbownik M, Reiter RJ, Garcia JJ, Cabrera J, Burkhardt S, Osuna C, Lewiński A. Indole-3-propionic acid, a melatonin-related molecule, protects hepatic microsomal membranes from iron-induced oxidative damage: relevance to cancer reduction. J Cell Biochem. 2001;81(3):507-13
- ↑ Reiter RJ, Tan DX, Osuna C, Gitto E. Actions of melatonin in the reduction of oxidative stress. A review. J Biomed Sci. 2000 Nov-Dec;7(6):444-58
- ↑ Reiter RJ, Guerrero JM, Garcia JJ, Acuña-Castroviejo D (November 1998). "Reactive oxygen intermediates, molecular damage, and aging. Relation to melatonin". Ann. N. Y. Acad. Sci. 854: 410–424. PMID 9928448. doi:10.1111/j.1749-6632.1998.tb09920.x.
