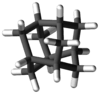
Iceane
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Tetracyclo[5.3.1.12,6.04,9]dodecane | |||
| Other names
Wurtzitane | |||
| Identifiers | |||
| 3D model (JSmol) |
|||
| ChemSpider | |||
| |||
| |||
| Properties | |||
| C12H18 | |||
| Molar mass | 163.56 g/mol | ||
| Structure | |||
| D3h | |||
| 0 D | |||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| | |||
| Infobox references | |||
Iceane is a saturated polycyclic hydrocarbon with formula C12H18. It has a cage-like molecular structure, whose carbon skeleton can be viewed as three fused cyclohexane rings in the boat conformation; or as two such rings in the chair conformation, connected by three parallel (axial) bonds.
The name "iceane" was proposed by the chemist Louis Fieser about a decade before the compound was first prepared. He was carrying out studies on the arrangement of water molecules in ice, when it occurred to him that there could exist a stable hydrocarbon with the above structure.[1][2]
It is also referred to as wurtzitane,[3] due to its similarity to the wurtzite crystal structure;[4] however, the name "iceane" has precedence.
See also
References
- ↑ Fieser, L. F. (1965). "Extensions in the use of plastic tetrahedral models". J. Chem. Educ. 42 (8): 408–412. doi:10.1021/ed042p408.
- ↑ Hargittai, M.; Hargittai, I. (2013). "Polyhedral Molecular Geometries". In Senechal, M. Shaping Space: Exploring Polyhedra in Nature, Art, and the Geometrical Imagination. Springer Science & Business Media. pp. 153–170,299. ISBN 9780387927145.
- ↑ Tobler, H.; Klaus, R. O.; Ganter, C. (1975). "Wurtzitan (Tetracyclo[5.3.1.12,6.04,9]dodecan)". Helv. Chim. Acta. 58 (5): 1455–1464. doi:10.1002/hlca.19750580522.
- ↑ Hamon, D. P. G.; Taylor, G. F. (1976). "A synthesis of tetracyclo[5,3,1,12,6,04,9]dodecane (iceane)". Aust. J. Chem. 29 (8): 1721–1734. doi:10.1071/CH9761721.
External links
- Royal Society of Chemistry Journal
- Symmetry Through the Eyes of a Chemist, Magdolna Hargittai
- A Basis for Synthesis Design, Tse-Lok Ho
- Structures and Energies of Polycyclic Hydrocarbons, Joan E. Shields
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.