Hydroxylamine-''O''-sulfonic acid
 | |
 | |
| Identifiers | |
|---|---|
| 3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.019.065 |
| EC Number | 220-971-6 |
| PubChem CID |
|
| |
| |
| Properties | |
| H3NO4S | |
| Molar mass | 113.09 |
| Appearance | white solid |
| Melting point | 210 °C |
| cold water | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Hydroxylamine-O-sulfonic acid ("HOSA") is the inorganic compound with molecular formula H3NO4S that is formed by the sulfonation of hydroxylamine with oleum.[1] It is a white, water-soluble and hygroscopic, solid, commonly represented by the condensed structural formula H2NOSO3H, though it actually exists as a zwitterion[2] and thus is more accurately represented as +H3NOSO3−. It is used as a reagent for the introduction of amine groups (–NH2), for the conversion of aldehydes into nitriles and alicyclic ketones into lactams (cyclic amides), and for the synthesis of variety of nitrogen-containing heterocycles.[2][3][4]
Preparation
According to a laboratory procedure[1] hydroxylamine-O-sulfonic acid can be prepared by treating hydroxylamine sulfate with fuming sulfuric acid (oleum). The industrial process is similar.[5]
![]()
The sulfonation of hydroxylamine can also be effected with chlorosulfonic acid[2] by a method first published in 1925[6] and refined for Organic Syntheses.[7]
Structure
Analogous to sulfamic acid (H3N+SO3−) and as is the case generally for amino acids, HOSA exists in the solid state as a zwitterion: H3N+OSO3−. It resembles an ammonia molecule coordinate covalently bonded to a sulfate group.[8]
Reactions
HOSA reacts under basic conditions as nucleophile and under neutral and acid conditions as electrophile.[3][9]
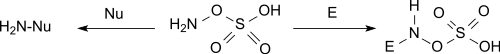
Aminations
It reacts with tertiary amines to trisubstituted hydrazinium salts and with pyridine to the 1-amino pyridinium salt.[10]

From 1-aminopyridinium salts the photochemically active 1-N-iminopyridinium ylides are accessible by acylation.[11] The photochemical rearrangement of the obtained 1-N-iminipyridinium ylides leads in high yields to 1H-1,2-diazepines[12]

N-amination of 1H-benzotriazole with hydroxylamine-O-sulfonic acid yields a mixture of 1-aminobenzotriazole (major product) and 2-aminobenzotriazole (minor product). From 1-aminotriazole, benzyne is formed in an almost quantitative yield by oxidation with lead(IV) acetate, which rapidly dimerizes to biphenylene in good yields.[13]

Electron deficient heterocycles, such as tetrazole, can be N-aminated with hydroxylamine-O-sulfonic acid, while even more electron-deficient compounds, such as 5-nitrotetrazole, react only with stronger aminating agents such as O-tosylhydroxylamine or O- mesitylene sulfonylhydroxylamine to amino compounds, which were investigated as explosives.[14]

In the N-amination of the unsubstituted tetrazole, a mixture of 1-amino- and 2-aminotetrazole is obtained.
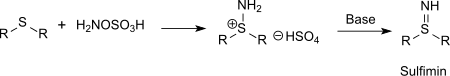
Also sulfur compounds (such as thioethers) can be aminated with hydroxylamine-O-sulfonic acid to sulfinimines (isosteric with sulfoxides but far more unstable) or phosphorus compounds (such as triphenylphosphine) can be aminated to phosphinimines via the intermediate aminotriphenylphosphonium hydrogen sulfate.[15]
The reaction of hydroxylamine-O-sulfonic acid with metal salts of sulfinic acids in sodium acetate solution produces primary sulfonamides in very good yields.[16]

Diimine can formed in situ from hydroxylamine-O-sulfonic acid respectively hydroxylamine-O-sulfonic acid hydroxylamine sulfate mixtures, which hydrogenates selectively conjugated multiple bonds.[20]
With carbonyl compounds
At room temperature and below, hydroxylamine-O-sulfonic acid reacts with ketones and aldehydes as a nucleophile to the corresponding oxime-O-sulfonic acids or their salts.[17] The oxime-O-sulfonic acids of aldehydes react above room temperature upon elimination of sulfuric acid in high yields to nitriles.[18]

Aliphatic ketones provide under similar conditions in very high yields oximes, arylalkyl ketones react in a Beckmann rearrangement to amides. When refluxed for several hours under acidic conditions (e.g. in the presence of concentrated methanoic acid) alicyclic ketones react to lactams in high yields.[19]

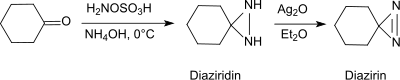
Under basic conditions in the presence of primary amines, hydroxylamine-O-sulfonic acid forms with aldehydes and ketones (e.g. cyclohexanone[20]) diaziridines, which can easily be oxidized to the more stable diazirines.
The reaction also provides substituted aziridines from simple aldehydes and ketones with high yield and diastereoselectivity.[21]

1,2-benzisoxazole is efficiently produced by nucleophilic attack of hydroxylamine-O-sulfonic acid to the carbonyl group of 2-hydroxybenzaldehyde followed by cyclization.[22]
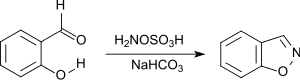
1,2-Benzisoxazole is a structural element in the antipsychotic risperidone and paliperidone, as well as the anticonvulsant zonisamide.
In a one-pot reaction, N-aryl[3,4-d]pyrazolopyrimidines are obtained in good yields from simple 4,6-dichloropyrimidine-5-carboxaldehyde,[23]

which can be used as purine analogues for a wide range of diagnostic and therapeutic applications.[24]
Further reactions
The chemiluminescence of the system luminol/cobalt(II) chloride is dramatically enhanced by the addition of hydroxylamine-O-sulfonic acid.[25]
References
- 1 2 Matsuguma, Harold J.; Audrieth, Ludwig F.; Wehrmeister, Herbert L. (1957). "Hydroxylamine-O-Sulfonic Acid". Inorg. Synth. 5: 122–125. doi:10.1002/9780470132364.ch32.
- 1 2 3 Wiberg, Egon; Wiberg, Nils (2001). "Sulfur Compounds of Nitrogen". Inorganic Chemistry. Academic Press. pp. 675–677. ISBN 978-0-12-352651-9.
- 1 2 Wallace, Raymond G. (1980). "Hydroxylamine-O-sulfonic acid – a versatile synthetic reagent". Aldrichimica Acta. 13 (1): 3–11.
- ↑ Rademacher, P. (2014). "Product Class 7: Hydrazines and Hydrazinium Salts (40.7.1.1.9.2 – Using Hydroxylamine-O-sulfonic Acids". In Enders, Dieter; Schaumann, E. Compounds with One Saturated Carbon–Heteroatom Bond: Amine N-Oxides, Haloamines, Hydroxylamines and Sulfur Analogues, and Hydrazines. Science of Synthesis: Houben-Weyl Methods of Molecular Transformations. 40b. Georg Thieme Verlag. p. 1171. ISBN 978-3-13-172181-5.
- ↑ US patent 3281209, Wehrmeister, Herbert L. & Harold I. Yalowitz, "Process for the preparation of hydroxylamine-O-sulfonic acid", published 1966-10-25, issued 1966-10-25, assigned to Commercial Solvents Corporation
- ↑ Sommer, F.; Schulz, O. F.; Nassau, M. (1925). "Über die Sulfoperamidsäure" [About Sulfoperamic Acid]. Z. Anorg. Allg. Chem. (in German). 147 (1): 142–155. doi:10.1002/zaac.19251470115.
- ↑ Rathke, Michael W.; Millard, Alan A. (1978). "Boranes in functionalization of olefins to amines: 3-Pinanamine (Bicyclo[3.1.1]heptan-3-amine, 2,6,6-trimethyl-)". Org. Synth. 58: 32. doi:10.15227/orgsyn.058.0032.; Coll. Vol., 6, p. 943
- ↑ Baenziger, Norman C.; Belt, Roger F.; Goebel, Carol V. (1967). "Crystal structure of hydroxylamine-O-sulfonic acid". Inorg. Chem. 6 (3): 511–514. doi:10.1021/ic50049a017.
- ↑ Erdik, Ender (2001). "Hydroxylamine-O-Sulfonic Acid". Encyclopedia of Reagents for Organic Synthesis. ISBN 0-471-93623-5. doi:10.1002/047084289X.rh058.
- ↑ R. Gösl; A. Meuwsen (1963). "1-Aminopyridinium iodide". Org. Synth. (in German). 43: 1. doi:10.15227/orgsyn.043.0001.
- ↑ J. Streith (1991). "The Photochemistry of N-Iminopyridinium Ylides in Retrospect. From a Simple Concept to Some Applications". CHIMIA (in German). 45 (3): 65–76.
- ↑ J. Streith (1977). "The photochemistry of aromatic-N-ylides. Rearrangement and fragmentation patterns". Pure & Appl. Chem. (in German). 49 (3): 305–315. doi:10.1351/pac197749030305.
- ↑ Campbell, C.D.; Rees, C.W. (1969). "Reactive intermediates. Part I. Synthesis and oxidation of 1- and 2-aminobenzotriazole". J. Chem. Soc. C. 1969 (5): 742–747. doi:10.1039/J39690000742.
- ↑ T.M. Klapötke; D.G. Piercey; J. Stierstorfer (2012). "Amination of energetic anions: high-performing energetic materials". Dalton Trans. (in German). 41 (31): 9451–9459. doi:10.1039/C2DT30684K.
- ↑ R. Appel; W. Büchner; E. Guth (1958). "Zur Kenntnis des Imins, I. Über Phosphinimine und Sulfinimine". Justus Liebigs Ann. Chem. (in German). 618 (1): 53–58. doi:10.1002/jlac.19586180107.
- ↑ S.L. Graham; T.H. Scholz (1986). "The reaction of sulfinic acid salts with hydroxylamine-O-sulfonic acid. A useful synthesis of primary sulfonamides". Synthesis (in German). 1986 (2): 1031–1032. doi:10.1055/s-1986-31862.
- ↑ J. Streith; C. Fizet (1977). "Nucleophilic versus electrophilic properties of the nitrogen atom in O-sulfonyl-hydroxylamine derivatives". Tetrahedron Lett. (in German). 18 (37): 3297–3300. doi:10.1016/S0040-4039(01)83223-8.
- ↑ C. Fizet; J. Streith (1974). "Hydroxylamine-O-sulfonic acid: A convenient reagent for the oxidative conversion of aldehydes into nitriles". Tetrahedron Lett. (in German). 15 (36): 3187–3188. doi:10.1016/S0040-4039(01)91857-X.
- ↑ G.A. Olah; A.P. Fung (1985). "Hexahydro-2-(1H)-azocinone". Org. Synth. (in German). 63: 188. doi:10.15227/orgsyn.063.0188.
- ↑ E. Schmitz; R. Ohme (1965). "3,3-Pentamethylenediaziridine". Org. Synth. (in German). 45: 83. doi:10.15227/orgsyn.045.0083.
- ↑ A.W. Beebe; E.F. Dohmeier; G. Moura-Letts (2015). "Diastereoselective synthesis of substituted diaziridines from simple ketones and aldehydes". Chem. Commun. (in German). 51 (70): 13511–13514. doi:10.1039/C5CC04813C.
- ↑ D.S. Kemp; R.B. Woodward (1965). "The N-ethylbenzisoxazolium cation—I : Preparation and reactions with nucleophilic species". Tetrahedron (in German). 21 (11): 3019–3035. doi:10.1016/S0040-4020(01)96921-2.
- ↑ L.E. Evans; M.D. Cheeseman; K. Jones (2012). "N–N Bond-Forming Cyclization for the One-Pot Synthesis of N-Aryl[3,4-d]pyrazolopyrimidines". Org. Lett. (in German). 14 (13): 3546–3549. doi:10.1021/ol301561a.
- ↑ C. Morrill; S. Babu; N.G. Almstead; Y.-C. Moon (2013). "Synthesis of 1,4-disubstituted pyrazolo[3,4-d]pyrimidines from 4,6-dichloropyrimidine-5-carboxaldehyde: insights into selectivity and reactivity". Synthesis (in German). 45 (13): 1791–1806. doi:10.1055/s-0033-1338862.
- ↑ M. Saqib; W. Gao; J. Lai; L. Qi; S. Majeed; M.R.H.S. Gilani; G. Xu (2015). "Hydroxylamine-O-sulfonic acid as an efficient coreactant for luminol chemiluminescence for selective and sensitive detection". Chem. Commun. (in German). 51 (30): 6536–6539. doi:10.1039/C5CC01090J.