Hydroxy group

A hydroxy or hydroxyl group is the entity with the formula OH. It contains oxygen bonded to hydrogen. In organic chemistry, alcohol and carboxylic acids contain hydroxy groups. The anion [OH−], called hydroxide, consists of a hydroxy group.
According to IUPAC rules, the term hydroxyl refers to the radical OH only, while the functional group −OH is called hydroxy group.[1]
Properties
Water, alcohols, carboxylic acids, and many other hydroxy-containing compounds can be deprotonated readily. This behavior is rationalized by the disparate electronegativities of oxygen and hydrogen. Hydroxy-containing compounds engage in hydrogen bonding, which causes them to stick together, leading to higher boiling and melting points than found for compounds that lack this functional group. Organic compounds, which are often poorly soluble in water, become water soluble when they contain two or more hydroxy groups, as illustrated by sugars and amino acid.
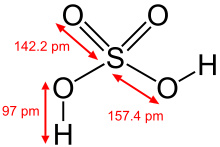
Occurrence
The hydroxy group is pervasive in chemistry and biochemistry. Many inorganic compounds contain hydroxy groups, including sulfuric acid, the chemical compound produced on the largest scale industrially.
Hydroxy groups participate in the dehydration reactions that link simple biological molecules into long chains. The joining of a fatty acid to glycerol to form a triacylglycerol removes the −OH from the carboxy end of the fatty acid. The joining of two aldehyde sugars to form a disaccharide removes the −OH from the carboxy group at the aldehyde end of one sugar. The creation of a peptide bond to link two amino acids to make a protein removes the −OH from the carboxy group of one amino acid.
Hydroxyl radical
Hydroxyl radicals are highly reactive and undergo chemical reactions that make them short-lived. When biological systems are exposed to hydroxyl radicals, they can cause damage to cells, including those in humans, where they react with DNA, lipids, and proteins.
Lunar and other extraterrestrial observations
In 2009, India's Chandrayaan-1 satellite, NASA's Cassini spacecraft and the Deep Impact probe have each detected the presence of water by evidence of hydroxyl fragments on the Moon. As reported by Richard Kerr, "A spectrometer [the Moon Mineralogy Mapper, a.k.a. "M3"] detected an infrared absorption at a wavelength of 3.0 micrometers that only water or hydroxyl—a hydrogen and an oxygen bound together—could have created."[2] NASA also reported in 2009 that the LCROSS probe revealed an ultraviolet emission spectrum consistent with hydroxyl presence.[3] The Venus Express orbiter sent back Venus science data from April 2006 until December 2014. Results from Venus Express include the detection of hydroxyl in the atmosphere.
See also
| Look up hydroxy group in Wiktionary, the free dictionary. |
| Wikimedia Commons has media related to Hydroxyl group. |
References
- ↑ "Alcohols". IUPAC. Retrieved 23 March 2015.
- ↑ Richard A. Kerr (24 September 2009). "A Whiff of Water Found on the Moon". Science Now. Retrieved 2016-06-01.
- ↑ Jonas Dino (13 November 2009). "LCROSS Impact Data Indicates Water on Moon". NASA. Retrieved 2009-11-14.
External links
- Reece, Jane; Urry, Lisa; Cain, Michael; Wasserman, Steven; Minorsky, Peter; Jackson, Robert (2011). "Unit 1, Chapter 4 &5." In Campbell Biology (9th ed.). Berge, Susan; Golden, Brandy; Triglia, Logan (eds.). San Francisco: Pearson Benjamin Cummings. ISBN 978-0-321-55823-7