Hydrosilylation
Hydrosilylation, also called catalytic hydrosilation, describes the addition of Si-H bonds across unsaturated bonds.[1] Ordinarily the reaction is conducted catalytically and usually the substrates are unsaturated organic compounds. Alkenes and alkynes give alkyl and vinyl silanes; aldehydes and ketones give silyl ethers. The process was first reported in academic literature in 1947.[2] Hydrosilylation has been called the "most important application of platinum in homogeneous catalysis."[3]
Scope and mechanism
The catalytic transformation represents an important method for preparing organosilicon compounds. An idealized transformation is the addition of triethylsilane to diphenylacetylene:[4]
- Et3SiH + PhC≡CPh → Et3Si(Ph)C=CH(Ph)
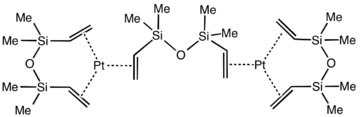 Kartstedt's catalyst is often used in hydrosilylation.
Kartstedt's catalyst is often used in hydrosilylation.
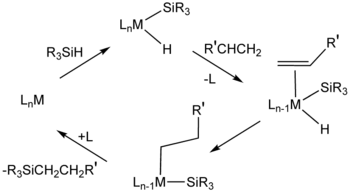
Hydrosilylation is related to hydrogenation, and similar catalysts are sometimes employed for the two catalytic processes. Popular industrial catalysts are "Speier's catalyst," H2PtCl6, and Karstedt’s catalyst, an alkene-stabilized platinum(0) catalyst.[5] One prevalent mechanism, called the Chalk-Harrod mechanism, assumes an intermediate metal complex that contains a hydride, a silyl ligand (R3Si), and the alkene substrate. The reaction usually produces anti-Markovnikov addition alkane, i.e., silicon on the terminal carbon.[1] Variations of the Chalk-Harrod mechanism. Some cases involve insertion of alkene into M-Si bond followed by reductive elimination, the opposite of the sequence in the Chalk-Harrod mechanism. In certain cases, hydrosilylation results in vinyl or allylic silanes resulting from beta-hydride elimination.[6]
These reactions can also be catalyzed using nanomaterial-based catalysts.
Asymmetric hydrosilylation
Using chiral phosphines as spectator ligands, catalysts have been developed for catalytic asymmetric hydrosilation. A well studied reaction is the addition of trichlorosilane to styrene to give 1-phenyl-1-(trichlorosilyl)ethane:
- Cl3SiH + PhCHCH2 → (Ph)(CH3)CHSiCl3
Nearly perfect enantioselectivities (ee's) can be achieved using palladium catalysts supported by binaphthyl-substituted monophosphine ligands.[7]
Surface hydrosilylation
Silicon wafers can be etched in hydrofluoric acid (HF) to remove the native oxide and form a hydrogen-terminated silicon surface. The hydrogen-terminated surfaces undergo hydrosilation with unsaturated compounds (such as terminal alkenes and alkynes), to form a stable monolayer on the surface. For example:
- Si-H + H2C=CH(CH2)7CH3 → Si-CH2CHH-(CH2)7CH3
The hydrosilylation reaction can be initiated with UV light at room temperature or with heat (typical reaction temperature 120-200 °C), under moisture- and oxygen-free conditions.[8] The resulting monolayer, which is stable and inert, inhibits oxidation of the base silicon layer, relevant to various device applications.[9]
References
- 1 2 "Hydrosilylation A Comprehensive Review on Recent Advances" B. Marciniec (ed.), Advances in Silicon Science, Springer Science, 2009. doi:10.1007/978-1-4020-8172-9
- ↑ Sommer, L.; Pietrusza, E.; Whitmore, F. "Peroxide-catalyzed addition of trichlorosilane to 1-octene". J. Amer. Chem. Soc. 69 (1): 188. doi:10.1021/ja01193a508.
- ↑ Renner, H.; Schlamp, G.; Kleinwächter, I.; Drost, E.; Lüschow, H. M.; Tews, P.; Panster, P.; Diehl, M.; Lang, J.; Kreuzer, T.; Knödler, A.; Starz, K. A.; Dermann, K.; Rothaut, J.; Drieselman, R. (2002). "Platinum group metals and compounds". Ullmann's Encyclopedia of Industrial Chemistry. Wiley. doi:10.1002/14356007.a21_075.
- ↑ James L. Fry, Ronald J. Rahaim Jr., Robert E. Maleczka Jr. "Triethylsilane", Encyclopedia of Reagents for Organic Synthesis, John Wiley & Sons, 2007. doi:10.1002/047084289X.rt226.pub2
- ↑ C. Elschenbroich, Organometallics (2006) Wiley and Sons-VCH: Weinheim. ISBN 978-3-527-29390-2
- ↑ Troegel, D.; Stohrer, J., "Recent advances and actual challenges in late transition metal catalyzed hydrosilylation of olefins from an industrial point of view", Coord. Chem. Rev. 2011, 255, 1440-1459. doi:10.1016/j.ccr.2010.12.025
- ↑ Hayashi, T.; Yamasaki, K. (2007). "C–E Bond Formation through Asymmetric Hydrosilylation of Alkenes". In Crabtree, Robert H.; Mingos, D. Michael P. Comprehensive Organometallic Chemistry III. Amsterdam: Elsevier. ISBN 978-0-08-045047-6. doi:10.1016/B0-08-045047-4/00140-0.
- ↑ "Photoreactivity of Unsaturated Compounds with Hydrogen-Terminated Silicon (111)," R. L. Cicero, M. R. Linford, C. E. D. Chidsey, Langmuir 16, 5688-5695 (2000).
- ↑ "Direct electrical detection of DNA Hybridization at DNA-modified silicon surfaces," W.Cai, J. Peck, D. van der Weide, and R.J. Hamers, Biosensors and Bioelectronics 19, 1013-1019 (2004).
Further reading
- Applied homogeneous catalysis with organometallic compounds : a comprehensive handbook : applications, developments. Boy Cornils; W A Herrmann. Publisher: Weinheim ; New York : Wiley-VCH, ©2000.
- Comprehensive handbook on hydrosilylation. Bogdan Marciniec. Publisher: Oxford [u.a.] : Pergamon Press, 1992.
- Rhodium complexes as hydrosilylation catalysts. N.K. Skvortsov. // Rhodium Express. 1994. No 4 (May). P. 3 - 36 (Eng). ISSN 0869-7876
Additional specialized literature
- "Alkyl Monolayers on Silicon Prepared from 1-Alkenes and Hydrogen-Terminated Silicon," M. R. Linford, P. Fenter, P. M. Eisenberger and C. E. D. Chidsey, J. Am. Chem. Soc. 117, 3145-3155 (1995).
- "Synthesis and characterization of DNA-modified Si(111) Surfaces," T. Strother, W. CAi, X. Zhao, R.J. Hamers, and L.M. Smith, J. Am. Chem. Soc. 122, 1205-1209 (2000).
- "T. Strother, R.J. Hamers, and L.M. Smith, "Surface Chemistry of DNA Covalent Attachment to the Silicon(100) Surface". Langmuir, 2002, 18, 788-796.
- "Covalently Modified Silicon and Diamond Surfaces: Resistance to Non-Specific Protein Adsorption and Optimization for Biosensing," T.L. Lasseter, B.H. Clare, N.L. Abbott, and R.J. Hamers. J. Am. Chem. Soc. 2004, 126, 10220-10221.