Bisulfite
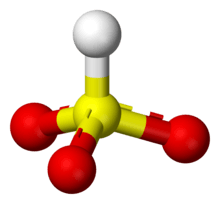
Bisulfite ion (IUPAC-recommended nomenclature: hydrogen sulfite) is the ion HSO3−. Salts containing the HSO3− ion are termed bisulfites also known as sulfite lyes. For example, sodium bisulfite is NaHSO3.
Structure
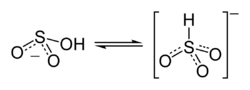

Some evidence may suggest that the proton in bisulfite ion is located on sulfur, giving rise to C3v symmetry. There is, however, some evidence from 17O NMR spectroscopy to suggest that two tautomeric forms of HSO3− exist in dynamic equilibrium, one which has the proton attached to sulfur (HSO3−) and one which is protonated at oxygen (HOSO2−).[1] The C3v structure is supported by X-ray crystallography and, in aqueous solution, by Raman spectroscopy (ν(S–H) = 2500 cm−1).
Reactions
Bisulfite salts are typically prepared by treatment of alkaline solutions with excess sulfur dioxide:
- SO2 + NaOH → NaHSO3
HSO3− is the conjugate base of sulfurous acid, H2SO3:
- H2SO3 ⇌ HSO3− + H+
Sulfurous acid is not an isolable compound and does not appear to exist in solution either. An equilibrium that is much more consistent with spectroscopic evidence is given :
- SO2 + H2O ⇌ HSO3− + H+
HSO3− is a weak acidic species with a pKa of 6.97. Its conjugate base is the sulfite ion, SO32−:
- HSO3− ⇌ SO32− + H+
Bisulfites are reducing agents, as are all sulfites and sulfur dioxide, which contains sulfur in the same oxidation state (+4).
Medicine
Bisulfite salts are common additives to the drug epinephrine in order to prevent its oxidation to adrenochrome and resulting inactivation. Bisulfites can sometimes cause an allergic reaction. This is different from the common sulfa drugs allergy. The quantity of bisulfite in medical pain blockers that initiates Type 1 hypersensitivity has been determined by Fraser and Huang.[2]
See also
References
- ↑ Horner, D. A.; Connick, R. E. (1986). "Equilibrium quotient for the isomerization of bisulfite ion from HSO3− to SO3H−". Inorganic Chemistry. 25 (14): 2414–2417. doi:10.1021/ic00234a026.
- ↑ Fraser, W. A.; Huang, A. S. (1984). "Are sulfites additives really safe?". New England Journal of Medicine. 311 (8): 542. doi:10.1056/NEJM198408233110824.