Host–parasite coevolution
| Part of a series on |
| Evolutionary biology |
|---|
 |
|
History of evolutionary theory |
|
Fields and applications
|
|
Host–parasite coevolution is a special case of coevolution, which is defined as the reciprocal adaptive genetic change of two antagonists (e.g. different species or genes) through reciprocal selective pressures. In the particular case of host–parasite coevolution the antagonists are different species of host and parasite.[1]
Introduction
Hosts and parasites exert reciprocal selective pressures on each other, which may lead to rapid reciprocal adaptation. For organisms with short generation times host–parasite coevolution can be observed in comparatively small time periods, making it possible to study evolutionary change in real-time under both field and laboratory conditions. These interactions may thus serve as a counter-example to the common notion that evolution can only be detected across extended time.
The high dynamics associated with these interactions are summarized in the Red Queen hypothesis. It states that "it takes all the running you can do to keep in the same place", i.e. both host and parasite have to change continuously to keep up with each other's adaptations.[2]
Host–parasite coevolution is ubiquitous and of potential importance to all living organisms, including humans, domesticated animals and crops. Major diseases such as malaria, AIDS and influenza are caused by coevolving parasites, and better understanding of coevolutionary adaptations between parasite attack strategies and host immune systems may assist in the development of novel medications and vaccines.[1]
Selection Dynamics
Host–parasite coevolution is characterized by reciprocal genetic change and thus changes in allele frequencies within populations. These changes may be determined by three main types of selection dynamics.
Negative frequency dependent selection

An allele is subject to negative frequency dependent selection if a rare allelic variant has a selective advantage. For example, the parasite should adapt to the most common host genotype, because it can then infect a large number of hosts. In turn, a rare host genotype may then be favored by selection, its frequency will increase and eventually it becomes common. Subsequently, the parasite should adapt to the former infrequent genotype.[3][4]
Coevolution determined by negative frequency dependent selection is rapid, potentially occurring across few generations.[3] It maintains high genetic diversity by favoring uncommon alleles. This selection mode is expected for multicellular hosts, because adaptations can occur without the need for novel advantageous mutations, which are less likely to be frequent in these hosts because of relatively small population sizes and relatively long generation times.[3]
Overdominant selection
Overdominance occurs if the heterozygote phenotype has a fitness advantage over both homozygotes ("heterozygote advantage" or "heterosis"). One example is sickle cell anemia. It is due to a mutation in the hemoglobin gene leading to sickle shape formation of red blood cells, causing clotting of blood vessels, restricted blood flow and reduced oxygen transport. At the same time, the mutation confers resistance to malaria, caused by Plasmodium parasites, which are passed off in red blood cells after transmission to humans by mosquitoes. Hence, homozygote and heterozygote genotypes for the sickle-cell disease allele show malaria resistance, while the homozygote suffers from severe disease phenotype. The alternative homozygote, which does not carry the sickle cell disease allele, is susceptible to Plasmodium. As a consequence, the heterozygote genotype is selectively favored in areas with a high incidence of malaria.
Directional selection

If an allele provides a fitness benefit, its frequency will increase within a population - selection is directional or positive. Selective sweeps are one form of directional selection, where the increase in frequency will eventually lead to the fixation of the advantageous allele. The process is considered to be slower in comparison to negative frequency dependent selection. It may produce an "arms race", consisting of the repeated origin and fixation of new parasite virulence and host defence traits.[1]
This mode of selection is likely to occur in interactions between unicellular organisms and viruses due to large population sizes, short generation times, often haploid genomes and horizontal gene transfer, which increase the probability of beneficial mutations arising and spreading through populations.[3]
Geographic Mosaic Theory of Coevolution
The Geographic Mosaic Theory of Coevolution by John N. Thompson hypothesizes that there is spatially divergent coevolutionary selection, producing genetic differentiation across populations.[5]
The model assumes that there are three crucial elements that jointly fuel ongoing coevolutionary change:[6][7][8]
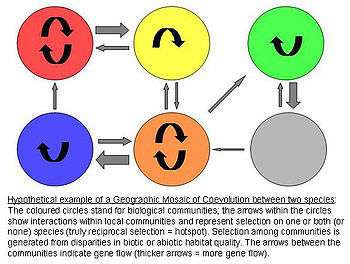
1) There is a selection mosaic among populations. Natural selection on interspecific interactions differs among populations. Thus genotype-by-genotype-by-environment (G x G x E) interactions affect fitness of the antagonists. In other words, the specific environmental conditions determine how any genotype of one species influences the fitness of another species.
2) There are coevolutionary hotspots. Coevolutionary hotspots are communities in which selection on the interaction is truly reciprocal. These hotspots are intermixed with so-called coldspots in which only one or neither species adapts to the antagonist.
3) There is a geographic mixing of traits. Between the communities/regions there is a continuous "mixing" of traits by gene flow, random genetic drift, population extinction, or mutations. This remixing determines the exact dynamics of the Geographic Mosaic by shifting the spatial distributions of potentially coevolving alleles and traits.
In summary, the theory claims that species interactions commonly coevolve as complex geographic mosaics of populations, formed by differences in local selection and gene flow. It ties together the processes operating over space and time to determine the outcome of coevolutionary interactions and furthermore provides a reference framework for future (especially field) research on this topic.[5]
Costs of adaptation
Resources are generally limited. Therefore, investment in one trait (e.g. virulence or immunity) limits investment in other life-history traits (e.g. reproductive rate). Moreoever, genes often have pleiotropic effects. Thus, a change in a pleiotropic immunity or virulence gene may automatically affect other traits. There is thus a trade-off between benefits and costs of the adaptive changes that then may prevent the host population to become fully resistant or the parasite population to express very high pathogenicity.
One example for costs related to gene pleiotropy was found for coevolving Escherichia coli and bacteriophages.[9]
To inject their genetic material, phages need to bind to a specific bacterial cell surface receptor. The bacterium may prevent injection by altering the relevant binding site, e.g. in response to point mutations or deletion of the receptor. However, these receptors have important functions in bacterial metabolism. Their loss would thus decrease fitness (i.e. growth rate). As a consequence, there is a trade-off between the advantages and disadvantages of a mutated receptor, leading to polymorphisms at this locus within bacterial populations coevolving with phages.
Examples
Potamopyrgus antipodarum and its trematode

The New Zealand freshwater snail Potamopyrgus antipodarum and its different trematode parasites represent a rather special model system. Populations of P. antipodarum consist of asexual clones and sexual individuals and therefore can be used to study the evolution and advantages of sexual reproduction. There is a high correlation between the presence of parasites and the frequency of sexual individuals within the different populations. This result is consistent with the Red Queen hypothesis that sexual reproduction is favoured during host–parasite coevolution.[10] At the same time, the persistence of sex may also rely on other factors, for example Muller's ratchet and/or the avoidance of the accumulation of deleterious mutations.
Caenorhabditis elegans and Bacillus thuringiensis

The nematode Caenorhabditis elegans and the bacterium Bacillus thuringiensis were only recently established as a model system for studying host–parasite coevolution. Laboratory evolution experiments provided evidence for many of the basic predictions of these coevolutionary interactions, including reciprocal genetic change, and increases in the rate of evolution and genetic diversity.[11]
Daphnia and its parasites

The crustacean Daphnia and its numerous parasites have become one of the main model systems for studying coevolution. One valuable characteristic of this system is that the reproduction of the host can be asexual as well as sexual (induced by changes in the external environment), so that conditions for sexual reproduction can be stimulated in the laboratory.[3] Coevolution was especially studied between Daphnia magna and the bacterium Pasteuria ramosa. For example, Decaestecker et al. reconstructed the evolution of the populations across decades, by reanimating resting stages of both species from laminated pond sediments and exposed hosts from each layer to parasites from the past, the same and the future layers. The study demonstrated that parasites were on average most infective on their contemporary hosts[12] in consistency with negative frequency dependent selection[13]
Tribolium castaneum and Nosema whitei

As many other arthropods Tribolium castaneum, the red flour beetle, is a host for the microsporidian Nosema whitei. Nosema whitei kills its host for transmission, thus the host’s lifespan is important for the parasite’s success. In turn, parasite fitness most likely depends on a trade-off between transmission (spore load) and virulence:[14] A higher virulence would increase the potential for the production of more offspring, but a higher spore load would affect the host’s lifespan and therefore the transmission rate. This trade-off is supported by coevolutionary experiments, which revealed the decrease of virulence, a constant transmission potential and an increase in the host’s lifespan over a period of time.[14] Further experiments demonstrated a higher recombination rate in the host during coevolutionary interactions, which may be selectively advantageous because it should increase diversity of host genotypes.[15]
Plantago and its parasites

Host–parasite coevolution is studied in a diversity of plant systems. One example is Plantago lanceolata and its parasite Podosphaera plantaginis, which has been intensively studied on the Aland islands in south-western Finland.[16] P. plantaginis is a powdery mildew obtaining nutrients from its host, a perennial herb, by sending feeding roots into the plant.
There are more than 3000 host populations known in this region, where both populations can evolve freely, in absence of human-imposed selection, in a very heterogeneous landscape. Both antagonists can reproduce asexually or sexually. This system was used to demonstrate spatially divergent coevolutionary dynamics across two metapopulations[17] and it provided support for the predictions of the Mosaic Theory of Coevolution.[5]
Bacteria and their parasites
Biological communities are often complex, they are influenced by diverse factors, and therefore, they are very difficult to study. A possible solution is to focus on simple community systems under controlled laboratory conditions, for example microbial communities consisting of Escherichia coli or Pseudomonas fluorescens and their bacteriophages.
E. coli, a Gram-negative proteobacterium, is a common model in biological research, for which comprehensive data on various aspects of its life-history is available. It has been used extensively for evolution experiments, including those related to coevolution with phages.[9] These studies revealed - among others - that coevolutionary adaptation may be influenced by pleiotropic effects of the involved genes. In particular, binding of the bacteriophage to E. coli surface receptor is the crucial step in the virus infection cycle. A mutation in the receptor's binding site may cause resistance. Such mutations often show pleiotropic effects and may cause a cost of resistance. In the presence of phages, such pleiotropy may lead to polymorphisms in the bacterial population and thus enhance biodiversity in the community. [9]
Another model system consists of the plant- and animal-colonizing bacterium Pseudomonas and its bacteriophages. This system provided new insights into the dynamics of coevolutionary change. It demonstrated that coevolution may proceed via recurrent selective sweeps, favouring generalists for both antagonists.[18][19] Furthermore, coevolution with phages may promote allopatric diversity, potentially enhancing biodiversity and possibly speciation.[20] Host-parasite coevolution may also affect the underlying genetics, for example by favouring increased mutation rates in the host.[21]
References
- 1 2 3 Woolhouse, M. E. J.; Webster, J. P.; Domingo, E.; Charlesworth, B.; Levin, B. R. (December 2002). "Biological and biomedical implications of the coevolution of pathogens and their hosts" (PDF). Nature Genetics. 32 (4): 569–77. PMID 12457190. doi:10.1038/ng1202-569.
- ↑ Rabajante, J; et al. (2016). "Host-parasite Red Queen dynamics with phase-locked rare genotypes". Science Advances. 2: e1501548. ISSN 2375-2548. doi:10.1126/sciadv.1501548.
- 1 2 3 4 5 Ebert, D (2008). "Host–parasite coevolution: Insights from the Daphnia–parasite model system". Current Opinion in Microbiology. 11 (3): 290–301. ISSN 1369-5274. PMID 18556238. doi:10.1016/j.mib.2008.05.012.
- ↑ Rabajante, J; et al. (2015). "Red Queen dynamics in multi-host and multi-parasite interaction system". Scientific Reports. 5: 10004. ISSN 2045-2322. PMC 4405699
 . PMID 25899168. doi:10.1038/srep10004.
. PMID 25899168. doi:10.1038/srep10004. - 1 2 3 Laine, Anna-Liisa (July 2009). "Role of coevolution in generating biological diversity - spatially divergent selection trajectories". Journal of Experimental Botany. 60 (11): 2957–2970. ISSN 1460-2431. PMID 19528527. doi:10.1093/jxb/erp168.
- ↑ JN Thompson Lab Homepage
- ↑ Thompson, John N. (2005). The Geographic Mosaic of Coevolution (Interspecific Interactions). University Of Chicago Press. ISBN 978-0-226-79762-5.
- ↑ Thompson, John N. (1999). "Specific Hypotheses on the Geographic Mosaic of Coevolution". The American Naturalist. 153: S1–S14. doi:10.1086/303208.
- 1 2 3 Bohannan, BJM; Lenski, RE (2000). "Linking genetic change to community evolution: insights from studies of bacteria and bacteriophage". Ecology Letters. 3: 362–77. ISSN 1461-0248. doi:10.1046/j.1461-0248.2000.00161.x.
- ↑ Jokela, Jukka; Liveley, Curtis M.; Dydahl, Mark F.; Fox, Jennifer A (7 May 2003). "Genetic variation in sexual and clonal lineages of a freshwater snail". Biological Journal of the Linnean Society. 79 (1): 165–181. ISSN 0024-4066. doi:10.1046/j.1095-8312.2003.00181.x.
- ↑ Schulte, RD; Makus C; Hasert B; Michiels NK; Schulenburg H (20 April 2010). "Multiple reciprocal adaptations and rapid genetic change upon experimental coevolution of an animal host and its microbial parasite" (PDF). PNAS. 107 (16): 7359–7364. ISSN 1091-6490. PMC 2867683
 . PMID 20368449. doi:10.1073/pnas.1003113107.
. PMID 20368449. doi:10.1073/pnas.1003113107. - ↑ Decaestecker, E; Gaba S; Raeymaekers JA; Stoks R; Van Kerckhoven L; Ebert D; De Meester L. (6 December 2007). "Host-parasite 'Red Queen' dynamics archived in pond sediment". Nature. 450 (7171): 870–3. ISSN 1476-4687. PMID 18004303. doi:10.1038/nature06291.
- ↑ Gandon, S; Buckling A; Decaestecker E; Day T (November 2008). "Host-parasite coevolution and patterns of adaptation across time and space". Journal of Evolutionary Biology. 21 (6): 1861–1866. ISSN 1010-061X. PMID 18717749. doi:10.1111/j.1420-9101.2008.01598.x.
- 1 2 Bérénos, C.; Schmid-Hempel P; Wegner, KM (October 2009). "Evolution of host resistance and trade-offs between virulence and transmission potential in an obligately killing parasite". Journal of Evolutionary Biology. 22 (10): 2049–56. ISSN 1420-9101. PMID 19732263. doi:10.1111/j.1420-9101.2009.01821.x.
- ↑ Fischer, O; Schmid-Hempel, P (2005). "Selection by parasites may increase host recombination frequency". Biology Letters. 22 (2): 193–5. doi:10.1098/rsbl.2005.0296.
- ↑ Soubeyrand S, Laine, A-L, Hanski I, Penttinen A (2009). "Spatio-temporal structure of host-pathogen interactions in a metapopulation" (PDF). The American Naturalist. 174 (3): 308–320. PMID 19627233. doi:10.1086/603624.
- ↑ Laine A-L (2005). Linking spatial and evolutionary dynamics in a plant-pathogen metapopulation. Department of Biological and Environmental Sciences, University of Helsinki, Finland, Academic dissertation
- ↑ Buckling, A; Rainey, PB (2002a). "Antagonistic coevolution between a bacterium and a bacteriophage" (PDF). Proceedings of the Royal Society: Biological Sciences. 269 (1494): 931–936. PMC 1690980
 . PMID 12028776. doi:10.1098/rspb.2001.1945.
. PMID 12028776. doi:10.1098/rspb.2001.1945. - ↑ Brockhurst, MA; Morgan, AD; Fenton, A; Buckling, A (2007). "Experimental coevolution with bacteria and phage: the Pseudomonas fluorescens model system" (PDF). Infection, Genetics and Evolution. 7: 547–552. doi:10.1016/j.meegid.2007.01.005.
- ↑ Buckling A, Rainey PB (2002b). "The role of parasites in sympatric and allopatric host diversification". Nature. 420 (6915): 496–499. PMID 12466840. doi:10.1038/nature01164. <http://www.nature.com/nature/journal/v420/n6915/pdf/nature01164.pdf>
- ↑ Pal C, Macia MD, Oliver A, Schachar I, Buckling A (2007). "Coevolution with viruses drives the evolution of bacterial mutation rates". Nature. 450 (7172): 1079–1081. PMID 18059461. doi:10.1038/nature06350. <http://www.nature.com/nature/journal/v450/n7172/pdf/nature06350.pdf>