Homarylamine
 | |
| Clinical data | |
|---|---|
| Routes of administration | Various |
| Identifiers | |
| |
| Synonyms |
1,3-benzodioxolyl-N-methyl-5-ethanamine; 3,4-methylenedioxy-N-methyl-2-phenylethylamine |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C10H13NO2 |
| Molar mass | 179.21572 g/mol |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
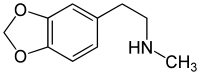
Homarylamine (INN;[1] also known as 3,4-methylenedioxy-N-methylphenethylamine or MDMPEA) is a substituted phenethylamine. It is the N-methylated analog of MDPEA.
Homoarylamine is known to have been patented for use as an antitussive agent.[2]
Derivatives
- Cyclization of Homoarylamine with formaldehyde gives Hydrastinine.
- Hydrastine
References
- ↑ "International Non-Proprietary Names for Pharmaceutical Preparations" (PDF). Chronicle of the World Health Organization. 12 (3). 1958.
- ↑ U.S. Patent 2,820,739
| Phenylalkyl- amines (other than cathinones) |
|
|---|---|
| Cyclized phenyl- alkylamines | |
| Cathinones | |
| Tryptamines | |
| Chemical classes | |
| Phenethylamines |
|
|---|---|
| Amphetamines |
|
| Phentermines |
|
| Cathinones |
|
| Phenylisobutylamines | |
| Phenylalkylpyrrolidines | |
| Catecholamines (and close relatives) |
|
| Miscellaneous |
|
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.