Hexahydro-1,3,5-triazine
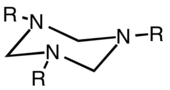
In chemistry, hexahydro-1,3,5-triazine is a class of heterocyclic compounds with the formula (CH2NR)3. They are reduced derivatives of 1,3,5-triazine, which have the formula (CHN)3, a family of aromatic heterocycles.
Preparation
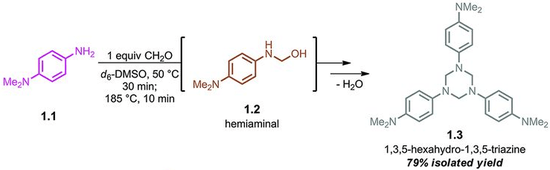
The parent hexahydro-1,3,5-triazine ((CH2NH)3) has been detected as an intermediate in the condensation of formaldehyde and ammonia, a reaction that affords hexamethylene tetraamine. The N-substituted derivatives are more stable. These N,N',N''-trisubstituted hexahydro-1,3,5-triazines arise from the condensation of the amine and formaldehyde as illustrated by the route to 1,3,5-trimethyl-1,3,5-triazacyclohexane:
- 3 CH2O + 3 H2NMe → (CH2NMe)3 + 3 H2O
The C-substituted derivatives are obtained by reaction of aldehydes and ammonia:[1]
- 3 RCHO + 3 H2NR → (RCHNH)3 + 3 H2O
Known as aldehyde ammonias, these compounds characteristically crystallize with water. 1-Alkanolamines are intermediates in these condensation reactions.[2]
The N,N,N-triacyl triazines arise from the reaction of hexamethylene tetraamine with acid chlorides or the condensation of amides with formaldehyde.[3]
Unlike the parent triazines, the hexahydro derivatives are conformationally flexible.[4] Trimers of isocyanates are sometimes labeled as 2,4,6-trioxohexahydro-1,3,5-triazines. They have the formula RNC(O))3. The explosive RDX is the trinitro derivative of hexahydro-1,3,5-triazine.
Hexahydro-1,3,5-triazine polymers have also been synthesized.[2]
References
- ↑ Nielsen, Arnold T.; Atkins, Ronald L.; Moore, Donald W.; Scott, Robert; Mallory, Daniel; LaBerge, Jeanne M. (1973). "Structure and chemistry of the aldehyde ammonias. 1-Amino-1-alkanols, 2,4,6-trialkyl-1,3,5-hexahydrotriazines, and N,N-dialkylidene-1,1-diaminoalkanes". J. Org. Chem. 38: 3288–3295. doi:10.1021/jo00959a010.
- 1 2 3 Garcia, J. M.; Jones, G. O.; Virwani, K.; McCloskey, B. D.; Boday, D. J.; Ter Huurne, G. M.; Horn, H. W.; Coady, D. J.; Bintaleb, A. M.; Alabdulrahman, A. M. S.; Alsewailem, F.; Almegren, H. A. A.; Hedrick, J. L. (2014). "Recyclable, Strong Thermosets and Organogels via Paraformaldehyde Condensation with Diamines". Science. 344 (6185): 732–5. PMID 24833389. doi:10.1126/science.1251484.
- ↑ Teeters, W. O.; Gradsten, M. A. (1950). "Hexahydro-1,3,5-tripropionyl-s-triazine". Org. Synth. 30: 51. doi:10.15227/orgsyn.030.0051.
- ↑ Jewett, J. G., Breeyear, J. J., Brown, J. H., Bushweller, C. H. (2000). "Stereodynamics of 1,3,5-Trialkyl-1,3,5-triazacyclohexanes: 1H and 13C Dynamic NMR Studies. Solvent Effects. Ab Initio and Molecular Mechanics Calculations". J. Am. Chem. Soc. 122: 308. doi:10.1021/ja990760d.