Hexafluoroacetone
 | |
 | |
| Names | |
|---|---|
| IUPAC name
1,1,1,3,3,3-hexafluoro- | |
| Other names
perfluoroacetone acetone hexafluoride perfluoro-2-propanone | |
| Identifiers | |
| 3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.010.616 |
| RTECS number | UC2450000 |
| |
| |
| Properties | |
| C3F6O | |
| Molar mass | 166.02 g/mol |
| Appearance | Colorless gas |
| Odor | musty[1] |
| Density | 1.32 g/ml, liquid |
| Melting point | −129 °C (144 K) |
| Boiling point | −28 °C (245 K) |
| Reacts with water | |
| Vapor pressure | 5.8 atm (20 °C)[1] |
| Hazards | |
| Main hazards | Toxic (T), Corrosive (C) |
| R-phrases (outdated) | R14, R23/24/25, R34, R60, R63 |
| S-phrases (outdated) | S7/9, S26, S28, S36, S45, S53 |
| NFPA 704 | |
| Flash point | Nonflammable[1] |
| US health exposure limits (NIOSH): | |
| PEL (Permissible) |
none[1] |
| REL (Recommended) |
TWA 0.1 ppm (0.7 mg/m3) [skin][1] |
| IDLH (Immediate danger) |
N.D.[1] |
| Related compounds | |
| Related ketones; organofluorides |
Acetone; Hexafluoro-2-propanol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
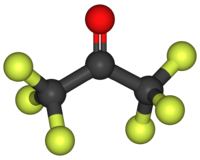
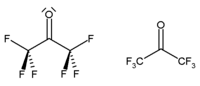
Hexafluoroacetone is a chemical compound with the formula CF3-CO-CF3. It is structurally similar to acetone; however, its reactivity is markedly different. It comes in the form of a colourless, hygroscopic, nonflammable, highly reactive gas characterized by a musty odour.[2] The most common form of this substance is hexafluoroacetone sesquihydrate (1.5 H2O), which is a geminal diol.
Synthesis and reactions
(CF3)2CO is prepared in a two step process from perfluoropropene. In the first step KF catalyzes the reaction of the alkene with elemental sulfur to give the 1,3-dithete [(CF3)2CS]2. This species is then oxidized by iodate to give (CF3)2CO.[3]
Uses
Hexafluoroacetone is mostly employed in organic synthesis, but it is also the main chemical intermediate used in the production of hexafluoroisopropanol, as well as polymethyl methacrylates and polyesters for textile coating. Hexafluoroacetone can be employed as a solvent for acetal resins, polyamides, polyglycolide, polyacetals, and polyols or as a polymer adhesive. Hexafluoroacetone is used in the synthesis of the sedative drug midaflur.
Reactivity
Hexafluoroacetone is a reactive substance, acting primarily as an electrophile. In water, hexafluoroacetone is highly reactive as its equilibrium sharply favors (~106) forming a geminal diol hydrate, unlike the identical unfavorable equilibrium with acetone (10−3).[4] This makes the equilibria of two chemicals differ by a factor of about a billion regarding this addition of water.[4] Hexafluoroacetone-hydrates are acidic and react with most metals to generate hydrogen. Hexafluoroacetone violently reacts in the presence of alkali. Related to its tendency to hydrate, (CF3)2CO adds ammonia to give (CF3)2C(OH)(NH2) which can be dehydrated with phosphoryl chloride to give (CF3)2CNH.[5]
See also
References
- 1 2 3 4 5 6 "NIOSH Pocket Guide to Chemical Hazards #0319". National Institute for Occupational Safety and Health (NIOSH).
- ↑ CDC - NIOSH Pocket Guide to Chemical Hazards
- ↑ Van Der Puy, M. ; Anello, L. G. (1990). "Hexafluoroacetone". Org. Synth.; Coll. Vol., 7, p. 251
- 1 2 Lemal, David M. (2004). "Perspective on Fluorocarbon Chemistry". The Journal of Organic Chemistry. 69 (1): 1–11. PMID 14703372. doi:10.1021/jo0302556.
- ↑ Middleton, W. J.; Carlson, H. D. (1988). "Hexafluoroacetoneimine". Org. Synth.; Coll. Vol., 6, p. 664
