GroEL
GroEL belongs to the chaperonin family of molecular chaperones, and is found in a large number of bacteria.[5] It is required for the proper folding of many proteins. To function properly, GroEL requires the lid-like cochaperonin protein complex GroES. In eukaryotes the proteins Hsp60 and Hsp10 are structurally and functionally nearly identical to GroEL and GroES, respectively.
Mechanism
Within the cell, the process of GroEL/ES mediated protein folding involves multiple rounds of binding, encapsulation, and release of substrate protein. Unfolded substrate proteins bind to a hydrophobic binding patch on the interior rim of the open cavity of GroEL, forming a binary complex with the chaperonin. Binding of substrate protein in this manner, in addition to binding of ATP, induces a conformational change that allows association of the binary complex with a separate lid structure, GroES. Binding of GroES to the open cavity of the chaperonin induces the individual subunits of the chaperonin to rotate such that the hydrophobic substrate binding site is removed from the interior of the cavity, causing the substrate protein to be ejected from the rim into the now largely hydrophilic chamber. The hydrophilic environment of the chamber favors the burying of hydrophobic residues of the substrate, inducing substrate folding. Hydrolysis of ATP and binding of a new substrate protein to the opposite cavity sends an allosteric signal causing GroES and the encapsulated protein to be released into the cytosol. A given protein will undergo multiple rounds of folding, returning each time to its original unfolded state, until the native conformation or an intermediate structure committed to reaching the native state is achieved. Alternatively, the substrate may succumb to a competing reaction, such as misfolding and aggregation with other misfolded proteins.[6]
Thermodynamics
The constricted nature of the interior of the molecular complex strongly favors compact molecular conformations of the substrate protein. Free in solution, long-range, non-polar interactions can only occur at a high cost in entropy. In the close quarters of the GroEL complex, the relative loss of entropy is much smaller. The method of capture also tends to concentrate the non-polar binding sites separately from the polar sites. When the GroEL non-polar surfaces are removed, the chance that any given non-polar group will encounter a non-polar intramolecular site are much greater than in bulk solution. The hydrophobic sites which were on the outside are gathered together at the top of the cis domain and bind each other. The geometry of GroEL requires that the polar structures lead, and they envelop the non-polar core as it emerges from the trans side.
Structure
Structurally, GroEL is a dual-ringed tetradecamer, with both the cis and trans rings consisting of seven subunits each. The conformational changes that occur within the central cavity of GroEL cause for the inside of GroEL to become hydrophilic, rather than hydrophobic, and is likely what facilitates protein folding.
 GroEL (side)
GroEL (side) GroEL (top)
GroEL (top) GroES/GroEL complex (side)
GroES/GroEL complex (side)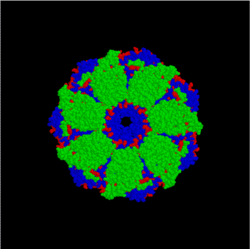 GroES/GroEL complex (top)
GroES/GroEL complex (top)
The key to the activity of GroEL is in the structure of the monomer. The Hsp60 monomer has three distinct sections separated by two hinge regions. The apical section contains a large number of hydrophobic binding sites for unfolded protein substrates. Many globular proteins won't bind to the apical domain because their hydrophobic parts are clustered inside, away from the aqueous medium since this is the thermodynamically optimal conformation. Thus, these "substrate sites" will only bind to proteins which are not optimally folded. The apical domain also has binding sites for the Hsp10 monomers of GroES.
The equatorial domain has a slot near the hinge point for binding ATP, as well as two attachment points for the other half of the GroEL molecule. The rest of the equatorial section is moderately hydrophilic.
The addition of ATP and GroES has a drastic effect on the conformation of the cis domain. This effect is caused by flexion and rotation at the two hinge points on the Hsp60 monomers. The intermediate domain folds down and inward about 25° on the lower hinge. This effect, multiplied through the cooperative flexing of all monomers, increases the equatorial diameter of the GroEL cage. But the apical domain rotates a full 60° up and out on the upper hinge, and also rotates 90° around the hinge axis. This motion opens the cage very widely at the top of the cis domain, but completely removes the substrate binding sites from the inside of the cage.
Interactions
GroEL has been shown to interact with GroES,[7][8] ALDH2,[8] Caspase 3[7][9] and Dihydrofolate reductase.[10]
See also
References
- 1 2 3 GRCh38: Ensembl release 89: ENSG00000144381 - Ensembl, May 2017
- 1 2 3 GRCm38: Ensembl release 89: ENSMUSG00000025980 - Ensembl, May 2017
- ↑ "Human PubMed Reference:".
- ↑ "Mouse PubMed Reference:".
- ↑ Zeilstra-Ryalls J, Fayet O, Georgopoulos C (1991). "The universally conserved GroE (Hsp60) chaperonins". Annu. Rev. Microbiol. 45: 301–25. PMID 1683763. doi:10.1146/annurev.mi.45.100191.001505.
- ↑ Horwich AL, Fenton WA, Chapman E, Farr GW (2007). "Two families of chaperonin: physiology and mechanism". Annu. Rev. Cell Dev. Biol. 23: 115–45. PMID 17489689. doi:10.1146/annurev.cellbio.23.090506.123555.
- 1 2 Samali A, Cai J, Zhivotovsky B, Jones DP, Orrenius S (Apr 1999). "Presence of a pre-apoptotic complex of pro-caspase-3, Hsp60 and Hsp10 in the mitochondrial fraction of jurkat cells". EMBO J. 18 (8): 2040–8. PMC 1171288
 . PMID 10205158. doi:10.1093/emboj/18.8.2040.
. PMID 10205158. doi:10.1093/emboj/18.8.2040. - 1 2 Lee KH, Kim HS, Jeong HS, Lee YS (Oct 2002). "Chaperonin GroESL mediates the protein folding of human liver mitochondrial aldehyde dehydrogenase in Escherichia coli". Biochem. Biophys. Res. Commun. 298 (2): 216–24. PMID 12387818. doi:10.1016/S0006-291X(02)02423-3.
- ↑ Xanthoudakis S, Roy S, Rasper D, Hennessey T, Aubin Y, Cassady R, Tawa P, Ruel R, Rosen A, Nicholson DW (Apr 1999). "Hsp60 accelerates the maturation of pro-caspase-3 by upstream activator proteases during apoptosis". EMBO J. 18 (8): 2049–56. PMC 1171289
 . PMID 10205159. doi:10.1093/emboj/18.8.2049.
. PMID 10205159. doi:10.1093/emboj/18.8.2049. - ↑ Mayhew M, da Silva AC, Martin J, Erdjument-Bromage H, Tempst P, Hartl FU (Feb 1996). "Protein folding in the central cavity of the GroEL-GroES chaperonin complex". Nature. 379 (6564): 420–6. PMID 8559246. doi:10.1038/379420a0.
Further reading
- Tabibzadeh S, Broome J (1999). "Heat shock proteins in human endometrium throughout the menstrual cycle". Infect Dis Obstet Gynecol. 7 (1-2): 5–9. PMC 1784709
 . PMID 10231001. doi:10.1002/(SICI)1098-0997(1999)7:1/2<5::AID-IDOG2>3.0.CO;2-Y.
. PMID 10231001. doi:10.1002/(SICI)1098-0997(1999)7:1/2<5::AID-IDOG2>3.0.CO;2-Y. - Schäfer C, Williams JA (2000). "Stress kinases and heat shock proteins in the pancreas: possible roles in normal function and disease". J. Gastroenterol. 35 (1): 1–9. PMID 10632533.
- Moseley P (2000). "Stress proteins and the immune response". Immunopharmacology. 48 (3): 299–302. PMID 10960671. doi:10.1016/S0162-3109(00)00227-7.
- Liu Y, Steinacker JM (2001). "Changes in skeletal muscle heat shock proteins: pathological significance". Front. Biosci. 6: D12–25. PMID 11145923. doi:10.2741/Liu.
- Van Maele B, Debyser Z (2005). "HIV-1 integration: an interplay between HIV-1 integrase, cellular and viral proteins". AIDS Rev. 7 (1): 26–43. PMID 15875659.
- Hochstrasser DF, Frutiger S, Paquet N, Bairoch A, Ravier F, Pasquali C, Sanchez JC, Tissot JD, Bjellqvist B, Vargas R (1992). "Human liver protein map: a reference database established by microsequencing and gel comparison". Electrophoresis. 13 (12): 992–1001. PMID 1286669. doi:10.1002/elps.11501301201.
- Ikawa S, Weinberg RA (1992). "An interaction between p21ras and heat shock protein hsp60, a chaperonin". Proc. Natl. Acad. Sci. U.S.A. 89 (6): 2012–6. PMC 48586
 . PMID 1347942. doi:10.1073/pnas.89.6.2012.
. PMID 1347942. doi:10.1073/pnas.89.6.2012. - Brudzynski K, Martinez V, Gupta RS (1992). "Immunocytochemical localization of heat-shock protein 60-related protein in beta-cell secretory granules and its altered distribution in non-obese diabetic mice". Diabetologia. 35 (4): 316–24. PMID 1516759. doi:10.1007/BF00401198.
- Dawson SJ, White LA (1992). "Treatment of Haemophilus aphrophilus endocarditis with ciprofloxacin". J. Infect. 24 (3): 317–20. PMID 1602151. doi:10.1016/S0163-4453(05)80037-4.
- Singh B, Patel HV, Ridley RG, Freeman KB, Gupta RS (1990). "Mitochondrial import of the human chaperonin (HSP60) protein". Biochem. Biophys. Res. Commun. 169 (2): 391–6. PMID 1972619. doi:10.1016/0006-291X(90)90344-M.
- Venner TJ, Singh B, Gupta RS (1990). "Nucleotide sequences and novel structural features of human and Chinese hamster hsp60 (chaperonin) gene families". DNA Cell Biol. 9 (8): 545–52. PMID 1980192. doi:10.1089/dna.1990.9.545.
- Ward LD, Hong J, Whitehead RH, Simpson RJ (1990). "Development of a database of amino acid sequences for human colon carcinoma proteins separated by two-dimensional polyacrylamide gel electrophoresis". Electrophoresis. 11 (10): 883–91. PMID 2079031. doi:10.1002/elps.1150111019.
- Jindal S, Dudani AK, Singh B, Harley CB, Gupta RS (1989). "Primary structure of a human mitochondrial protein homologous to the bacterial and plant chaperonins and to the 65-kilodalton mycobacterial antigen". Mol. Cell. Biol. 9 (5): 2279–83. PMC 363030
 . PMID 2568584.
. PMID 2568584. - Waldinger D, Eckerskorn C, Lottspeich F, Cleve H (1988). "Amino-acid sequence homology of a polymorphic cellular protein from human lymphocytes and the chaperonins from Escherichia coli (groEL) and chloroplasts (Rubisco-binding protein)". Biol. Chem. Hoppe-Seyler. 369 (10): 1185–9. PMID 2907406. doi:10.1515/bchm3.1988.369.2.1185.
- Kreisel W, Hildebrandt H, Schiltz E, Köhler G, Spamer C, Dietz C, Mössner W, Heilmann C (1994). "Immuno-gold electron microscopical detection of heat shock protein 60 (hsp60) in mitochondria of rat hepatocytes and myocardiocytes". Acta Histochem. 96 (1): 51–62. PMID 7518175. doi:10.1016/s0065-1281(11)80009-7.
- Corbett JM, Wheeler CH, Baker CS, Yacoub MH, Dunn MJ (1994). "The human myocardial two-dimensional gel protein database: update 1994". Electrophoresis. 15 (11): 1459–65. PMID 7895732. doi:10.1002/elps.11501501209.
- Baca-Estrada ME, Gupta RS, Stead RH, Croitoru K (1994). "Intestinal expression and cellular immune responses to human heat-shock protein 60 in Crohn's disease". Dig. Dis. Sci. 39 (3): 498–506. PMID 7907543. doi:10.1007/BF02088334.
- Vélez-Granell CS, Arias AE, Torres-Ruíz JA, Bendayan M (1994). "Molecular chaperones in pancreatic tissue: the presence of cpn10, cpn60 and hsp70 in distinct compartments along the secretory pathway of the acinar cells". J. Cell. Sci. 107 (3): 539–49. PMID 7911805.
- Mayhew M, da Silva AC, Martin J, Erdjument-Bromage H, Tempst P, Hartl FU (1996). "Protein folding in the central cavity of the GroEL-GroES chaperonin complex". Nature. 379 (6564): 420–6. PMID 8559246. doi:10.1038/379420a0.
- Tabibzadeh S, Kong QF, Satyaswaroop PG, Babaknia A (1996). "Heat shock proteins in human endometrium throughout the menstrual cycle". Hum. Reprod. 11 (3): 633–40. PMID 8671282. doi:10.1093/humrep/11.3.633.
External links
- GroEL Protein at the US National Library of Medicine Medical Subject Headings (MeSH)
- "Palaeos Bacteria: Pieces: GroEL". (No rights reserved)
- 3D macromolecular structures of GroEL in EMDB







