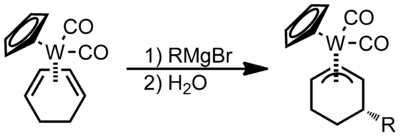Green–Davies–Mingos rules
In organometallic chemistry, the Green–Davies–Mingos rules predict the regiochemistry for nucleophilic addition to 18-electron metal complexes containing multiple unsaturated ligands.[1] In general, complexation enhances the susceptibility of unsaturated hydrocarbon toward nucleophilic attack. The rules were developed by Stephen G. Davies, Malcolm Green, and Michael Mingos in 1978 [2]
Rule 1
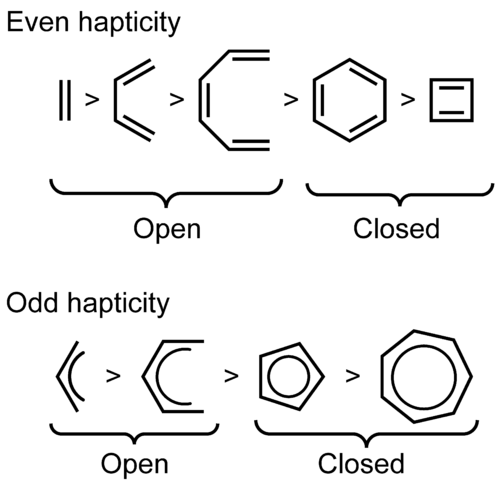
Nucleophilic attack is preferred on even-numbered polyenes (even hapticity).
Rule 2
Nucleophiles preferentially add to acyclic polyenes rather than cyclic polyenes.
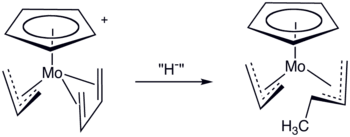
Rule 3
Nucleophiles preferentially add to even-hapticity polyene ligands at a terminus.[2] Nucleophiles add to odd-hapticity acyclic polyene ligands at a terminal position if the metal is highly electrophilic, otherwise they add at an internal site.
Simplified: even before odd and open before closed
The following is a diagram showing the reactivity trends of even/odd hapticity and open/closed pi-ligands.
The metal center is electron withdrawing. This effect is enhanced if the metal is also attached to a carbonyl. Electron poor metals do not back bond well to the carbonyl. The more electron withdrawing the metal is, the more triple bond character the CO ligand has. This gives the ligand a higher force constant. The resultant force constant found for a ligated carbonyl represents the same force constant for pi ligands if they replaced the CO ligand in the same complex.
Nucleophilic addition does not occur if kCO* (the effective force constant for the CO ligand) is below a threshold value [3]
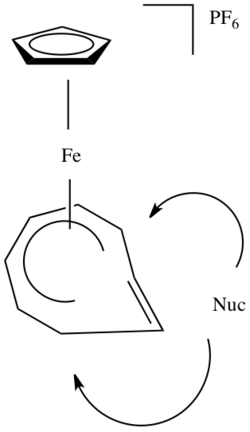
The following figure shows a ligated metal attached to a carbonyl group. This group has a partial positive charge and therefore is susceptible to nucleophilic attack. If the ligand represented by Ln were a pi-ligand, it would be activated toward nucleophilic attack as well.
Incoming nucleophilic attack happens at one of the termini of the pi-system in the figure below:
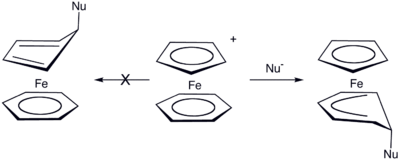
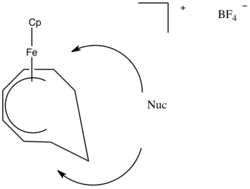
In this example the ring system can be thought of as analogous to butadiene. Following the Green–Davies–Mingos rules, since butadiene is an open pi-ligand of even hapticity, nucleophilic attack will occur at one of the terminal positions of the pi-system. This occurs because the LUMO of butadiene has larger lobes on the ends rather than the internal positions.
Examples of complexes
The following is an example of a complex containing three types of pi-ligands, demonstrating the preferential attack of a nucleophile at one of the pi-systems.

The above complex contains three types of pi-ligands. The cyclooctane ring contains a butadiene fragment on the left and an allyl fragment on the right. A cyclopentadiene ligand on the cobalt center gives the third type.
Attack of the cyanide nucleophile occurs preferentially at a terminus of the butadiene fragment. (The drawing above shows the incorrect product)
Following the rules given above, the butadiene fragment is an open ligand of even hapticity, which has greater reactivity than the allyl fragment, an open ligand of odd hapticity, or the cyclopentadiene, a closed ligand of odd hapticity.
Attack occurs at the terminus which will result in the conjugated product shown.
Internal attack
Here the ligand that is already attached to the metal acts as the nucleophile and attacks the metal center internally.[4][5]

Effects of types of ligands on regiochemistry of attack
Nucleophilic attack at terminal position of allyl ligands when pi accepting ligand is present.[6]
If sigma donating ligands are present they pump electrons into the ligand and attack occurs at the internal position.

Effects of asymmetrical ligands
When asymmetrical allyl ligands are present attack occurs at the more substituted position.[7]
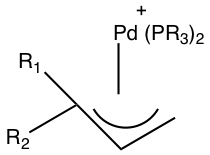
In this case the attack will occur on the carbon with both R groups attached to it since that is the more substituted position.
Effects of large pi ligands
When large pi ligands [8] are present they can undergo different kinds of nucleophilic attacks. In the following figure the nucleophilic attack can occur from either the top or bottom and reduce the double bond and add the nucleophile.
This nucleophilic attack can occur at either the top or bottom also and add the nucelophile.
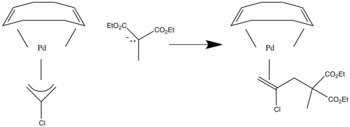
Uses in synthesis
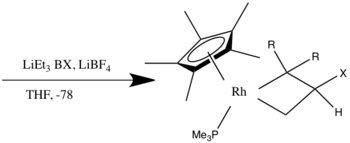
Nucleophilic addition to pi ligands can be used in synthesis. One example of this is to make cyclic metal compounds.[9] Nucleophiles add to the center of the pi ligand and produces a metallobutane.

References
- ↑ Stephen G. Davies; et al. (1977). "Nucleophilic Addition to Organotransition Metal Cations Containing Unsaturated Hydrocarbon Ligands". Tetrahedron. 34: 3047–3077. doi:10.1016/0040-4020(78)87001-X.
- 1 2 S.G. Davies, M. L. H. Green, and D. M. P. Mingos, Tetrahedron 1978, 34, 3047
- ↑ R. C. Bush and R. J. Angelici, J. Am. Chem. Soc. 1986, 108, 2133
- ↑ R. A. Periana and R. G. Bergman, J. Am. Chem. Soc. 1984, 106, 7272
- ↑ T. Suzuki, G. Okada, Y. Hioki, and H. Fujimoto, Organometallics 2003, 22, 3649
- ↑ A. Aranyos, K. J. Szabo, A. M. Castano, and J.-E. Backvall. Organometallics 1997, 16, 1056
- ↑ F. Delbecq and C. Lapouge, Organometallics 2000, 19, 2716
- ↑ S. Scho ̈rshusen and J. Heck, Organometallics 2007, 26, 5386
- ↑ R. Periana and R. Bergman, J. Am. Chem. Soc. 1986, 108, 7346