Glyoxylic acid
 | |
 | |
| Names | |
|---|---|
| IUPAC name
oxoethanoic acid | |
| Other names
formylformic acid; oxoethanoic acid | |
| Identifiers | |
| 3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.005.508 |
| KEGG | |
| PubChem CID |
|
| |
| |
| Properties | |
| C2H2O3 | |
| Molar mass | 74.04 g·mol−1 |
| Density | 1.384 g/mL |
| Melting point | 80 °C (176 °F; 353 K)[1] |
| Boiling point | 111 °C (232 °F; 384 K) |
| Acidity (pKa) | 3.18,[2] 3.32 [3] |
| Related compounds | |
| Other anions |
glyoxylate |
| Related carboxylic acids |
formic acid acetic acid glycolic acid oxalic acid propionic acid pyruvic acid |
| Related compounds |
acetaldehyde glyoxal glycolaldehyde |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Glyoxylic acid or oxoacetic acid is an organic compound. Together with acetic acid, glycolic acid, and oxalic acid, glyoxylic acid is one of the C2 carboxylic acids. It is a colourless solid that occurs naturally and is useful industrially.
Structure and nomenclature
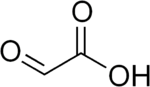
Glyoxylic acid is usually described with the chemical formula OCHCO2H, i.e. containing an aldehyde functional group (see image in upper right). The aldehyde in fact is not observed in solution or as a solid. As seen for many other aldehydes, it exists most commonly as the hydrate. Thus, the formula for glyoxylic acid is really (HO)2CHCO2H, described as the "monohydrate." This geminal diol exists in equilibrium with the dimeric hemiacetal in solution:[4] Henry's law constant of glyoxylic acid is KH = 1.09 × 104 × exp[(40.0 × 103/R) × (1/T − 1/298)].[5]
- 2 (HO)2CHCO2H ⇌ O[(HO)CHCO2H]2 + H2O
Preparations
The conjugate base of gloxylic acid is known as glyoxylate and is the form that the compound exists in solution at neutral pH. Glyoxylate is the byproduct of the amidation process in biosynthesis of several amidated peptides.
The compound is formed by organic oxidation of glyoxal with hot nitric acid, the main side product being oxalic acid.
Ozonolysis of maleic acid is also effective.[4]
Historically glyoxylic acid was prepared from oxalic acid electrosynthetically:[6][7]
In organic synthesis, lead dioxide anodes were applied for the production of glyoxylic acid from oxalic acid in a sulfuric acid electrolyte.[8]
Biological Role
Glyoxylate is an intermediate of the glyoxylate cycle, which enables organisms, such as bacteria,[9] fungi, and plants [10] to convert fatty acids into carbohydrates. The glyoxylate cycle is also important for induction of plant defense mechanisms in response to fungi.[11] The glyoxylate cycle is initiated through the activity of isocitrate lyase, which converts isocitrate into glyoxylate and succinate. Research is being done to co-opt the pathway for a variety of uses such as the biosynthesis of succinate.[12]
Glyoxylate in Humans
Glyoxylate is produced via two pathways: through the oxidation of glycolate in peroxisomes or through the catabolism of hydroxyproline in mitochondria.[13] In the peroxisomes, glyoxylate is converted into glycine by AGT1 or into oxalate by glycolate oxidase. In the mitochondria, glyoxylate is converted into glycine by AGT2 or into glycolate by glycolate reductase. A small amount of glyoxylate is converted into oxalate by cytoplasmic lactate dehydrogenase.[14]

Glyoxylate in Plants
In addition to being an intermediate in the glyoxylate pathway, glyoxylate is also an important intermediate in the photorespiration pathway. Photorespiration is a result of the side reaction of Rubisco with O2 instead of CO2. While at first considered a waste of energy and resources, photorespiration has been shown to be an important method of regenerating carbon and CO2, removing toxic phosphoglycolate, and initiating defense mechanisms.[15][16] In photorespiration, glyoxylate is converted from glycolate through the activity of glycolate oxidase in the peroxisome. It is then converted into glycine through parallel actions by SGAT and GGAT, which is then transported into the mitochondria.[17][16] It has also been reported that the pyruvate dehydrogenase complex may play a role in glycolate and glyoxylate metabolism.[18]

Disease Relevance
Diabetes
Glyoxylate is thought to be a potential early marker for Type II diabetes.[19] One of the key conditions of diabetes pathology is the production of advanced glycation end-products (AGEs) caused by the hyperglycemia.[20] AGEs can lead to further complications of diabetes, such as tissue damage and cardiovascular disease.[21] They are generally formed from reactive aldehydes, such as those present on reducing sugars and alpha-oxoaldehydes. In a study, glyoxylate levels were found to be significantly increased in patients who were later diagnosed with Type II diabetes.[19] The elevated levels were found sometimes up to three years before the diagnosis, demonstrating the potential role for glyoxylate to be an early predictive marker.
Nephrolithiasis
Glyoxylate is involved in the development of hyperoxaluria, a key cause of nephrolithiasis (commonly known as kidney stones). Glyoxylate is both a substrate and inductor of sulfate anion transporter-1 (sat-1), a gene responsible for oxalate transportation, allowing it to increase sat-1 mRNA expression and as a result oxalate efflux from the cell. The increased oxalate release allows the buildup of calcium oxalate in the urine, and thus the eventual formation of kidney stones.[14]
The disruption of glyoxylate metabolism provides an additional mechanism of hyperoxaluria development. Loss of function mutations in the HOGA1 gene leads to a loss of the 4-hydroxy-2-oxoglutarate aldolase, an enzyme in the hydroxyproline to glyoxylate pathway. The glyoxylate resulting from this pathway is normally stored away to prevent oxidation to oxalate in the cytosol. The disrupted pathway, however, causes a buildup of 4-hydroxy-2-oxoglutarate which can also be transported to the cytosol and converted into glyoxylate through a different aldolase. These glyoxylate molecules can be oxidized into oxalate increasing its concentration and causing hyperoxaluria.[13]
Reactions and uses
Glyoxylic acid is about 10x stronger acid than acetic acid, with an acid dissociation constant of 4.7 × 10−4 (pKa = 3.32):
- OCHCO2H ⇌ OCHCO2− + H+
With base, glyoxylic acid disproportionates:
- 2 OCHCO2H + H2O → HOCH2CO2H + (CO2H)2
Glyoxylic acid gives heterocycles upon condensation with urea and 1,2-diaminobenzene.
Phenol derivatives
Its condensation with phenols is versatile. The immediate product is 4-hydroxymandelic acid. This species reacts with ammonia to give hydroxyphenylglycine, a precursor to the drug amoxicillin. Reduction of the 4-hydroxymandelic acid gives 4-hydroxyphenylacetic acid, a precursor to the drug atenolol. Condensations with guaiacol in place of phenol provides a route to vanillin, a net formylation.[4][22][23]
Hopkins Cole reaction
Glyoxylic acid is a component of the Hopkins Cole reaction, used to check for the presence of tryptophan in proteins.
Safety
The compound is not very toxic with an LD50 for rats of 2500 mg/kg.
References
- ↑ Merck Index, 11th Edition, 4394
- ↑ Dissociation Constants Of Organic Acids and Bases (600 compounds), http://zirchrom.com/organic.htm.
- ↑ pKa Data Compiled by R. Williams, http://research.chem.psu.edu/brpgroup/pKa_compilation.pdf.
- 1 2 3 Georges Mattioda and Yani Christidis “Glyoxylic Acid” Ullmann's Encyclopedia of Industrial Chemistry, 2002, Wiley-VCH, Weinheim. doi:10.1002/14356007.a12_495
- ↑ Ip, H. S. Simon; Huang, X. H. Hilda; Yu, Jian Zhen. "Effective Henry's law constants of glyoxal, glyoxylic acid, and glycolic acid". Geophysical Research Letters. 36 (1). doi:10.1029/2008GL036212.
- ↑ Tafel, Julius; Friedrichs, Gustav (1904). "Elektrolytische Reduction von Carbonsäuren und Carbonsäureestern in schwefelsaurer Lösung". Berichte der deutschen chemischen Gesellschaft. 37 (3): 3187–3191. doi:10.1002/cber.190403703116. Retrieved 19 December 2013.
- ↑ Cohen, Julius (1920). Practical Organic Chemistry 2nd Ed. (PDF). London: Macmillan and Co. Limited. pp. 102–104.
- ↑ François Cardarelli (2008). Materials Handbook: A Concise Desktop Reference. Springer. p. 574. ISBN 1-84628-668-9.
- ↑ Holms WH (1987). "Control of flux through the citric acid cycle and the glyoxylate bypass in Escherichia coli". Biochem Soc Symp. 54: 17–31. PMID 3332993.
- ↑ Escher CL, Widmer F (1997). "Lipid mobilization and gluconeogenesis in plants: do glyoxylate cycle enzyme activities constitute a real cycle? A hypothesis". Biol Chem. 378 (8): 803–813. PMID 9377475.
- ↑ Dubey, Mukesh K.; Broberg, Anders; Sooriyaarachchi, Sanjeewani; Ubhayasekera, Wimal; Jensen, Dan Funck; Karlsson, Magnus (2013-09). "The glyoxylate cycle is involved in pleotropic phenotypes, antagonism and induction of plant defence responses in the fungal biocontrol agent Trichoderma atroviride". Fungal Genetics and Biology. 58–59: 33–41. ISSN 1087-1845. doi:10.1016/j.fgb.2013.06.008. Retrieved 2017-03-09. Check date values in:
|date=(help) - ↑ Zhu, Li-Wen; Li, Xiao-Hong; Zhang, Lei; Li, Hong-Mei; Liu, Jian-Hua; Yuan, Zhan-Peng; Chen, Tao; Tang, Ya-Jie (2013-11). "Activation of glyoxylate pathway without the activation of its related gene in succinate-producing engineered Escherichia coli". Metabolic Engineering. 20: 9–19. ISSN 1096-7176. doi:10.1016/j.ymben.2013.07.004. Retrieved 2017-03-09. Check date values in:
|date=(help) - 1 2 Belostotsky, Ruth; Pitt, James Jonathon; Frishberg, Yaacov (2012-12-01). "Primary hyperoxaluria type III—a model for studying perturbations in glyoxylate metabolism". Journal of Molecular Medicine. 90 (12): 1497–1504. ISSN 0946-2716. doi:10.1007/s00109-012-0930-z. Retrieved 2017-03-09.
- 1 2 Schnedler, Nina; Burckhardt, Gerhard; Burckhardt, Birgitta C. (2011-03). "Glyoxylate is a substrate of the sulfate-oxalate exchanger, sat-1, and increases its expression in HepG2 cells". Journal of Hepatology. 54 (3): 513–520. ISSN 0168-8278. doi:10.1016/j.jhep.2010.07.036. Retrieved 2017-03-09. Check date values in:
|date=(help) - ↑ "photorespiration". Retrieved 2017-03-09.
- 1 2 Peterhansel, Christoph; Horst, Ina; Niessen, Markus; Blume, Christian; Kebeish, Rashad; Kürkcüoglu, Sophia; Kreuzaler, Fritz (2010-03-23). "Photorespiration". The Arabidopsis Book / American Society of Plant Biologists. 8. ISSN 1543-8120. PMC 3244903
 . PMID 22303256. doi:10.1199/tab.0130.
. PMID 22303256. doi:10.1199/tab.0130. - ↑ Zhang, Zhisheng; Mao, Xingxue; Ou, Juanying; Ye, Nenghui; Zhang, Jianhua; Peng, Xinxiang (2015-01). "Distinct photorespiratory reactions are preferentially catalyzed by glutamate:glyoxylate and serine:glyoxylate aminotransferases in rice". Journal of Photochemistry and Photobiology B: Biology. 142: 110–117. ISSN 1011-1344. doi:10.1016/j.jphotobiol.2014.11.009. Retrieved 2017-03-09. Check date values in:
|date=(help) - ↑ Blume, Christian; Behrens, Christof; Eubel, Holger; Braun, Hans-Peter; Peterhansel, Christoph (2013-11). "A possible role for the chloroplast pyruvate dehydrogenase complex in plant glycolate and glyoxylate metabolism". Phytochemistry. 95: 168–176. ISSN 0031-9422. doi:10.1016/j.phytochem.2013.07.009. Retrieved 2017-03-09. Check date values in:
|date=(help) - 1 2 Nikiforova, Victoria J.; Giesbertz, Pieter; Wiemer, Jan; Bethan, Bianca; Looser, Ralf; Liebenberg, Volker; Ruiz Noppinger, Patricia; Daniel, Hannelore; Rein, Dietrich (2014). "Glyoxylate, a New Marker Metabolite of Type 2 Diabetes". Journal of Diabetes Research. 2014. ISSN 2314-6745. PMC 4265698
 . PMID 25525609. doi:10.1155/2014/685204.
. PMID 25525609. doi:10.1155/2014/685204. - ↑ Nguyen, Dung V.; Shaw, Lynn C.; Grant, Maria B. (2012-12-21). "Inflammation in the pathogenesis of microvascular complications in diabetes". Frontiers in Endocrinology. 3. ISSN 1664-2392. PMC 3527746
 . PMID 23267348. doi:10.3389/fendo.2012.00170.
. PMID 23267348. doi:10.3389/fendo.2012.00170. - ↑ Piarulli, Francesco; Sartore, Giovanni; Lapolla, Annunziata (2013-04). "Glyco-oxidation and cardiovascular complications in type 2 diabetes: a clinical update". Acta Diabetologica. 50 (2): 101–110. ISSN 0940-5429. PMC 3634985
 . PMID 22763581. doi:10.1007/s00592-012-0412-3. Check date values in:
. PMID 22763581. doi:10.1007/s00592-012-0412-3. Check date values in: |date=(help) - ↑ Fatiadi, Alexander; Schaffer, Robert (1974). "An Improved Procedure for Synthesis of DL-4-Hydroxy-3-methoxymandelic Acid (DL-"Vanillyl"-mandelic Acid, VMA)" (PDF). Journal of Research of the National Bureau of Standards Section A. 78A (3): 411–412. doi:10.6028/jres.078A.024. Retrieved 19 December 2013.
- ↑ Kamlet, Jonas; Mathieson, Olin (1953). Manufacture of vanillin and its homologues U.S. Patent 2,640,083 (PDF). U.S. Patent Office.
