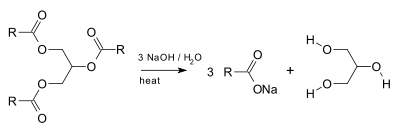Glycerol
 | |||
| |||
 | |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Propane-1,2,3-triol | |||
| Other names
Glycerin Glycerine Propanetriol 1,2,3-Trihydroxypropane 1,2,3-Propanetriol | |||
| Identifiers | |||
| 3D model (JSmol) |
|||
| ChEBI | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.000.263 | ||
| E number | E422 (thickeners, ...) | ||
| KEGG | |||
| PubChem CID |
|||
| UNII | |||
| |||
| |||
| Properties | |||
| C3H8O3 | |||
| Molar mass | 92.09 g·mol−1 | ||
| Appearance | colorless liquid hygroscopic | ||
| Odor | odorless | ||
| Density | 1.261 g/cm3 | ||
| Melting point | 17.8 °C (64.0 °F; 290.9 K) | ||
| Boiling point | 290 °C (554 °F; 563 K)[1] | ||
| miscible[2] | |||
| Vapor pressure | 0.003 mmHg (50°C)[2] | ||
| -57.06·10−6 cm3/mol | |||
| Refractive index (nD) |
1.4746 | ||
| Viscosity | 1.412 Pa·s[3] | ||
| Pharmacology | |||
| A06AG04 (WHO) A06AX01 (WHO), QA16QA03 (WHO) | |||
| Hazards | |||
| Safety data sheet | See: data page JT Baker | ||
| NFPA 704 | |||
| Flash point | 160 °C (320 °F; 433 K) (closed cup) 176 °C (349 °F; 449 K) (open cup) | ||
| US health exposure limits (NIOSH): | |||
| PEL (Permissible) |
TWA 15 mg/m3 (total) TWA 5 mg/m3 (resp)[2] | ||
| REL (Recommended) |
None established[2] | ||
| IDLH (Immediate danger) |
N.D.[2] | ||
| Supplementary data page | |||
| Refractive index (n), Dielectric constant (εr), etc. | |||
| Thermodynamic data |
Phase behaviour solid–liquid–gas | ||
| UV, IR, NMR, MS | |||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| | |||
| Infobox references | |||
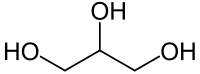
Glycerol (/ˈɡlɪsərɒl/;[4] also called glycerine or glycerin; see spelling differences) is a simple polyol compound. It is a colorless, odorless, viscous liquid that is sweet-tasting and non-toxic. The glycerol backbone is found in all lipids known as triglycerides. It is widely used in the food industry as a sweetener and humectant and in pharmaceutical formulations. Glycerol has three hydroxyl groups that are responsible for its solubility in water and its hygroscopic nature.[5]
Structure
Although achiral, glycerol is prochiral with respect to reactions of one of the two primary alcohols. Thus, in substituted derivatives, the stereospecific numbering labels each carbon as either sn-1, sn-2, or sn-3.[6][7]
Production
Glycerol is generally obtained from plant and animal sources where it occurs as triglycerides. Triglycerides are esters of glycerol with long-chain carboxylic acids. The hydrolysis, saponification, or transesterification of these triglycerides produces glycerol as well as the fatty acid derivative:
Triglycerides (1) are treated with an alcohol such as ethanol (2) with catalytic base to give ethyl esters of fatty acids (3) and glycerol (4):
Typical plant sources include soybeans or palm. Animal-derived tallow is another source. Approximately 950,000 tons per year are produced in the United States and Europe; 350,000 tons of glycerol were produced per year in the United States alone from 2000 to 2004.[8] Production will increase as the EU directive 2003/30/EC are implemented, which required the replacement of 5.75% of petroleum fuels with biofuel across all member states by 2010, as glycerol is a byproduct in the production of biodiesel. It was projected in 2006 that by the year 2020, production would be six times more than demand.[5]
Glycerol from triglycerides is produced on a large scale, but the crude product is of variable quality, with a low selling price of as low as 2-5 U.S. cents per kilogram in 2011.[9] It can be purified, but the process is expensive. Some glycerol is burned for energy, but its heat value is low.[10]
Crude glycerol from the hydrolysis of triglycerides can be purified by treatment with activated carbon to remove organic impurities, alkali to remove unreacted glycerol esters, and ion exchange to remove salts. High purity glycerol (> 99.5%) is obtained by multi-step distillation; vacuum is helpful due to the high boiling point of glycerol (290 °C).[5]
Synthetic glycerol
Although usually not cost-effective, glycerol can be produced by various routes from propylene. The epichlorohydrin process is the most important; it involves the chlorination of propylene to give allyl chloride, which is oxidized with hypochlorite to dichlorohydrins, which reacts with a strong base to give epichlorohydrin. This epichlorohydrin is then hydrolyzed to give glycerol. Chlorine-free processes from propylene include the synthesis of glycerol from acrolein and propylene oxide.[5]
Because of the large-scale production of biodiesel from fats, where glycerol is a waste product, the market for glycerol is depressed. Thus, synthetic processes are not economical. Owing to oversupply, efforts are being made to convert glycerol to synthetic precursors, such as acrolein and epichlorohydrin.[11] (See the Chemical intermediate section of this article.)
Applications
Food industry
In food and beverages, glycerol serves as a humectant, solvent, and sweetener, and may help preserve foods. It is also used as filler in commercially prepared low-fat foods (e.g., cookies), and as a thickening agent in liqueurs. Glycerol and water are used to preserve certain types of plant leaves.[12] As a sugar substitute, it has approximately 27 kilocalories per teaspoon (sugar has 20) and is 60% as sweet as sucrose. It does not feed the bacteria that form plaques and cause dental cavities. As a food additive, glycerol is labeled as E number E422. It is added to icing (frosting) to prevent it from setting too hard.
As used in foods, glycerol is categorized by the Academy of Nutrition and Dietetics as a carbohydrate. The U.S. Food and Drug Administration (FDA) carbohydrate designation includes all caloric macronutrients excluding protein and fat. Glycerol has a caloric density similar to table sugar, but a lower glycemic index and different metabolic pathway within the body, so some dietary advocates accept glycerol as a sweetener compatible with low-carbohydrate diets.
It is also recommended as an additive when using polyol sweeteners such as erythritol and xylitol which have a cooling effect, due to its heating effect in the mouth, if the cooling effect is not wanted.[13]
Pharmaceutical and personal care applications

Glycerol is used in medical, pharmaceutical and personal care preparations, mainly as a means of improving smoothness, providing lubrication, and as a humectant. It is found in allergen immunotherapies, cough syrups, elixirs and expectorants, toothpaste, mouthwashes, skin care products, shaving cream, hair care products, soaps, and water-based personal lubricants. In solid dosage forms like tablets, glycerol is used as a tablet holding agent. For human consumption, glycerol is classified by the U.S. FDA among the sugar alcohols as a caloric macronutrient.
Glycerol is a component of glycerin soap. Essential oils are added for fragrance. This kind of soap is used by people with sensitive, easily irritated skin because it prevents skin dryness with its moisturizing properties. It draws moisture up through skin layers and slows or prevents excessive drying and evaporation.
Glycerol can be used as a laxative when introduced into the rectum in suppository or small-volume (2–10 ml) (enema) form; it irritates the anal mucosa and induces a hyperosmotic effect.[14]
Taken orally (often mixed with fruit juice to reduce its sweet taste), glycerol can cause a rapid, temporary decrease in the internal pressure of the eye. This can be useful for the initial emergency treatment of severely elevated eye pressure.[15]
Botanical extracts
When utilized in "tincture" method extractions, specifically as a 10% solution, glycerol prevents tannins from precipitating in ethanol extracts of plants (tinctures). It is also used as an "alcohol-free" alternative to ethanol as a solvent in preparing herbal extractions. It is less extractive when utilized in a standard tincture methodology. Alcohol-based tinctures can also have the alcohol removed and replaced with glycerol for its preserving properties. Such products are not "alcohol-free" in a scientific sense, as glycerol contains three hydroxyl groups. Fluid extract manufacturers often extract herbs in hot water before adding glycerol to make glycerites.[16][17]
When used as a primary "true" alcohol-free botanical extraction solvent in non-tincture based methodologies, glycerol has been shown to possess a high degree of extractive versatility for botanicals including removal of numerous constituents and complex compounds, with an extractive power that can rival that of alcohol and water–alcohol solutions. That glycerol possesses such high extractive power assumes it is utilized with dynamic methodologies as opposed to standard passive "tincturing" methodologies that are better suited to alcohol. Glycerol possesses the intrinsic property of not denaturing or rendering a botanical's constituents inert (as alcohols – i.e. ethyl (grain) alcohol, methyl (wood) alcohol, etc., do). Glycerol is a stable preserving agent for botanical extracts that, when utilized in proper concentrations in an extraction solvent base, does not allow inverting or reduction-oxidation of a finished extract's constituents, even over several years. Both glycerol and ethanol are viable preserving agents. Glycerol is bacteriostatic in its action, and ethanol is bactericidal in its action.[18][19][20]
Electronic cigarette liquid
Vegetable glycerin is a common component of e-liquid, a solution used with electronic vaporizers (electronic cigarettes). This glycerol is heated with an atomizer (a heating coil often made of Kanthal wire), producing the aerosol that delivers nicotine to the user. [21]
Antifreeze
Like ethylene glycol and propylene glycol, glycerol is a non-ionic kosmotrope that forms strong hydrogen bonds with water molecules, competing with water-water hydrogen bonds. This disrupts the crystal lattice formation of ice unless the temperature is significantly lowered. The minimum freezing point temperature is about −36 °F (−38 °C) corresponding to 70% glycerol in water.
Glycerol was historically used as an anti-freeze for automotive applications before being replaced by ethylene glycol, which has a lower freezing point. While the minimum freezing point of a glycerol-water mixture is higher than an ethylene glycol-water mixture, glycerol is not toxic and is being re-examined for use in automotive applications.[22][23]
In the laboratory, glycerol is a common component of solvents for enzymatic reagents stored at temperatures below 0 °C due to the depression of the freezing temperature. It is also used as a cryoprotectant where the glycerol is dissolved in water to reduce damage by ice crystals to laboratory organisms that are stored in frozen solutions, such as bacteria, nematodes, and mammalian embryos.
Internal combustion fuel
Glycerol is also used to power diesel generators supplying electricity for the FIA Formula E series of electric race cars.[24]
Chemical intermediate
Glycerol is used to produce nitroglycerin, which is an essential ingredient of various explosives such as dynamite, gelignite, and propellants like cordite. Reliance on soap-making to supply co-product glycerol made it difficult to increase production to meet wartime demand. Hence, synthetic glycerol processes were national defense priorities in the days leading up to World War II. Nitroglycerin, also known as glyceryl trinitrate (GTN) is commonly used to relieve angina pectoris, taken in the form of sub-lingual tablets, or as an aerosol spray.
Allyl iodide, a chemical building block for polymers, preservatives, organometallic catalysts, and pharmaceuticals, can be synthesized by using elemental phosphorus and iodine on glycerol.[25]
A great deal of research is being conducted to try to make value-added products from crude glycerol (typically containing 20% water and residual esterification catalyst) obtained from biodiesel production.[26] The use of crude glycerol as an additive to biomass for a renewable energy source when burned or gasified is also being explored.
- Hydrogen gas production unit[27]
- Glycerine acetate (as a potential fuel additive)[28]
- Conversion to propylene glycol[29]
- Conversion to acrolein[30][31]
- Conversion to ethanol[32]
- Conversion to epichlorohydrin,[33] a raw material for epoxy resins
Film industry
Glycerol is used by the film industry when filming scenes involving water to stop areas from drying out too quickly.[34]
Ultrasonic couplant
Glycerol can be sometimes used as replacement for water in ultrasonic testing, as it has favourably higher acoustic impedance (2.42MRayl vs 1.483MRayl for water) while being relatively safe, non-toxic, non-corrosive and relatively low cost.[35]
Vibration dampening
Glycerol is used as fill for pressure gauges to dampen vibration. External vibrations, from compressors, engines, pumps, etc., produce harmonic vibrations within Bourdon gauges that can cause the needle to move excessively, giving inaccurate readings. The excessive swinging of the needle can also damage internal gears or other components, causing premature wear. Glycerol, when poured into a gauge to replace the air space, reduces the harmonic vibrations that are transmitted to the needle, increasing the lifetime and reliability of the gauge.[36]
Metabolism
Glycerol is a precursor for synthesis of triacylglycerols and of phospholipids in the liver and adipose tissue. When the body uses stored fat as a source of energy, glycerol and fatty acids are released into the bloodstream. Circulating glycerol does not glycate proteins as do glucose or fructose, and does not lead to the formation of advanced glycation endproducts (AGEs). In some organisms, the glycerol component can enter the glycolysis pathway directly and, thus, provide energy for cellular metabolism (or, potentially, be converted to glucose through gluconeogenesis).
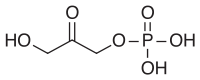
Before glycerol can enter the pathway of glycolysis or gluconeogenesis (depending on physiological conditions), it must be converted to their intermediate glyceraldehyde 3-phosphate in the following steps:
| Glycerol | Glycerol kinase | Glycerol-3-phosphate | Glycerol-3-phosphate dehydrogenase | Dihydroxyacetone phosphate | Triosephosphate isomerase | Glyceraldehyde 3-phosphate | |||
| |
ATP | ADP | |
NAD+ | NADH |
 |
 | ||
 |
 |
 | |||||||
| NAD+ | NADH |
||||||||
The enzyme glycerol kinase is present mainly in the liver and kidneys, but also in other body tissues, including muscle and brain.[37][38][39] In adipose tissue, glycerol 3-phosphate is obtained from dihydroxyacetone phosphate (DHAP) with the enzyme glycerol-3-phosphate dehydrogenase.
Glycerol has very low toxicity when ingested; its LD50 oral dose for rats is 12600 mg/kg and 8700 mg/kg for mice.
Historical cases of contamination with diethylene glycol
On 4 May 2007, the US Food and Drug Administration advised all US makers of medicines to test all batches of glycerol for the toxic diethylene glycol.[40] This followed an occurrence of hundreds of fatal poisonings in Panama resulting from a falsified import customs declaration by Panamanian import/export firm Aduanas Javier de Gracia Express, S. A. The cheaper diethylene glycol was relabeled as the more expensive glycerol.[41][42]
Etymology
The origin of the gly- and glu- prefixes for glycols and sugars is from Greek γλυκύς glukus which means sweet.[43]
Isomers
- 1,1,1-Propanetriol
- 1,1,2-Propanetriol
- 1,1,3-Propanetriol
- 1,2,2-Propanetriol
See also
- Epichlorohydrin
- Nitroglycerin
- Oleochemicals
- Saponification/Soapmaking
- Solketal
- Sugar alcohol
- Transesterification
References
- ↑ Lide, D. R., ed. (1994). CRC Handbook of Data on Organic Compounds (3rd ed.). Boca Raton, FL: CRC Press. p. 4386.
- 1 2 3 4 5 "NIOSH Pocket Guide to Chemical Hazards #0302". National Institute for Occupational Safety and Health (NIOSH).
- ↑ Segur, J. B.; Oberstar, H. E. (1951). "Viscosity of Glycerol and Its Aqueous Solutions". Industrial & Engineering Chemistry. 43 (9): 2117–2120. doi:10.1021/ie50501a040.
- ↑ Oxford dictionary: definition of glycerol (British & World English)
- 1 2 3 4 Christoph, Ralf; Schmidt, Bernd; Steinberner, Udo; Dilla, Wolfgang; Karinen, Reetta (2006). "Glycerol". Ullmann's Encyclopedia of Industrial Chemistry. Ullmann's Encyclopedia of Industrial Chemistry. ISBN 3527306730. doi:10.1002/14356007.a12_477.pub2.
- ↑ Hirschmann, H. (1960-10-01). "The Nature of Substrate Asymmetry in Stereoselective Reactions". Journal of Biological Chemistry. 235 (10): 2762–2767. PMID 13714619.
- ↑ "IUPAC-IUB Commission on Biochemical Nomenclature (CBN)". European Journal of Biochemistry. 2 (2): 127–131. 1967-09-01. doi:10.1111/j.1432-1033.1967.tb00116.x.
- ↑ Nilles, Dave (2005). "A Glycerin Factor". Biodiesel Magazine.
- ↑ Pei San Kong, Mohamed Kheireddine Aroua, Wan Mohd Ashri Wan Daud, "Conversion of crude and pure glycerol into derivatives: A feasibility evaluation", Renewable and Sustainable Energy Reviews, 2016, Volume 63, pp. 533-555. doi:10.1016/j.rser.2016.05.054
- ↑ Sims, Bryan (25 October 2011). "Clearing the way for byproduct quality: why quality for glycerin is just as important for biodiesel". Biodiesel Magazine.
- ↑ Yu, Bin (2014). "Glycerol". Synlett. 25 (4): 601–602. doi:10.1055/s-0033-1340636.
- ↑ Stevens, Alan. "Preserving flowers and decorative foliages with glycerin and dye" (PDF).
- ↑ Nikolov, Ivan. "Functional Food Design Rules".
- ↑ "Glycerin Enema". Drugs.com. Retrieved 17 November 2012.
- ↑ "Glycerin (Oral Route)". Mayo Foundation for Medical Education and Research. Retrieved 17 November 2012.
- ↑ Long, Walter S. (14 January 1916 – 13 January 1917). "The composition of commercial fruit extracts". Transactions of the Kansas Academy of Science. 28: 157–161. JSTOR 3624347. doi:10.2307/3624347.
- ↑ Does alcohol belong in herbal tinctures? newhope.com
- ↑ Lawrie, James W. (1928) Glycerol and the glycols – production, properties and analysis. The Chemical Catalog Company, Inc., New York, NY.
- ↑ Leffingwell, Georgia and Lesser, Miton (1945) Glycerin – its industrial and commercial applications. Chemical Publishing Co., Brooklyn, NY.
- ↑ The manufacture of glycerol – Vol. III (1956). The Technical Press, LTD., London, UK.
- ↑ "Electronic cigarette". Wikipedia. 2017-05-07.
- ↑ Hudgens, R. Douglas; Hercamp, Richard D.; Francis, Jaime; Nyman, Dan A.; Bartoli, Yolanda (2007). "An Evaluation of Glycerin (Glycerol) as a Heavy Duty Engine Antifreeze/Coolant Base". SAE Technical Paper Series. SAE Technical Paper Series. 1. doi:10.4271/2007-01-4000.
- ↑ Proposed ASTM Engine Coolant Standards Focus on Glycerin. Astmnewsroom.org. Retrieved on 15 August 2012.
- ↑ Formula E uses pollution-free glycerine to charge cars. fiaformulae.com. 13 September 2014
- ↑ Datta, Rasek Lal (1914). "The Preparation of Allyl Iodide". Journal of the American Chemical Society. 36 (5): 1005–1007. doi:10.1021/ja02182a023.
- ↑ Johnson, Duane T.; Taconi, Katherine A. (2007). "The glycerin glut: Options for the value-added conversion of crude glycerol resulting from biodiesel production". Environmental Progress. 26 (4): 338–348. doi:10.1002/ep.10225.
- ↑ Marshall, A. T.; Haverkamp, R. G. (2008). "Production of hydrogen by the electrochemical reforming of glycerol-water solutions in a PEM electrolysis cell". International Journal of Hydrogen Energy. 33 (17): 4649–4654. doi:10.1016/j.ijhydene.2008.05.029.
- ↑ Melero, Juan A.; Van Grieken, Rafael; Morales, Gabriel; Paniagua, Marta (2007). "Acidic mesoporous silica for the acetylation of glycerol: Synthesis of bioadditives to petrol fuel". Energy Fuels. 21 (3): 1782–1791. doi:10.1021/ef060647q.
- ↑ "Dow achieves another major milestone in its quest for sustainable chemistries" (Press release). Dow Chemical Company. 15 March 2007.
- ↑ Ott, L.; Bicker, M.; Vogel, H. (2006). "The catalytic dehydration of glycerol in sub- and supercritical water: a new chemical process for acrolein production". Green Chemistry. 8 (2): 214–220. doi:10.1039/b506285c.
- ↑ Watanabe, Masaru; Iida, Toru; Aizawa, Yuichi; Aida, Taku M.; Inomata, Hiroshi (2007). "Acrolein synthesis from glycerol in hot-compressed water". Bioresource Technology. 98 (6): 1285–1290. PMID 16797980. doi:10.1016/j.biortech.2006.05.007.
- ↑ Yazdani, S. S.; Gonzalez, R. (2007). "Anaerobic fermentation of glycerol: a path to economic viability for the biofuels industry". Current Opinion in Biotechnology. 18 (3): 213–219. PMID 17532205. doi:10.1016/j.copbio.2007.05.002. Lay summary – ScienceDaily (27 June 2007).
- ↑ "Dow Epoxy advances glycerine-to-epichlorohydrin and liquid epoxy resins projects by choosing Shanghai site" (Press release). Dow Chemical Company. 26 March 2007.
- ↑ Chemicals in Film. reagent.co.uk
- ↑ Acoustic Properties for Liquids. nde-ed.org
- ↑ Pneumatic Systems: Principles and Maintenance by S. R. Majumdar -- McGraw-Hill 2006 Page 74
- ↑ Tildon, J. T.; Stevenson Jr, J. H.; Ozand, P. T. (1976). "Mitochondrial glycerol kinase activity in rat brain". The Biochemical Journal. 157 (2): 513–6. PMC 1163884
 . PMID 183753. doi:10.1042/bj1570513.
. PMID 183753. doi:10.1042/bj1570513. - ↑ Newsholme, E. A.; Taylor, K (May 1969). "Glycerol kinase activities in muscles from vertebrates and invertebrates". Biochem. J. 112 (4): 465–74. PMC 1187734
 . PMID 5801671. doi:10.1042/bj1120465.
. PMID 5801671. doi:10.1042/bj1120465. - ↑ Jenkins, BT, Hajra, AK (1976). "Glycerol Kinase and Dihydroxyacetone Kinase in Rat Brain". Journal of Neurochemistry. 26 (2): 377–385. PMID 3631. doi:10.1111/j.1471-4159.1976.tb04491.x.
- ↑ "FDA Advises Manufacturers to Test Glycerin for Possible Contamination". U.S. Food and Drug Administration. 4 May 2007. Retrieved 8 May 2007.
- ↑ Walt Bogdanich (6 May 2007). "From China to Panama, a Trail of Poisoned Medicine". New York Times. Retrieved 8 May 2007.
- ↑ "10 Biggest Medical Scandals in History". 20 February 2013.
- ↑ glyco-, dictionary.com