GlnA RNA motif
| glnA RNA motif | |
|---|---|
|
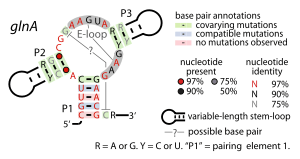
Consensus secondary structure of glnA RNAs | |
| Identifiers | |
| Symbol | glnA RNA |
| Rfam | RF01739 |
| Other data | |
| RNA type | Cis-regulatory element; riboswitch |
| Domain(s) | cyanobacteria |
| GO | GO:0070406 (glutamine binding); |
| SO | SO:0000035 (riboswitch); |
The glnA RNA motif is a conserved RNA structure that was predicted by bioinformatics.[1] It is present in a variety of lineages of cyanobacteria, as well as some phages that infect cyanobacteria. It is also found in DNA extracted from uncultivated bacteria living in the ocean that are presumably species of cyanobacteria.
glnA RNAs are found in the presumed 5' untranslated regions of genes encoding multiple classes of protein that are involved in nitrogen metabolism. The most prominent of these protein classes are ammonium transporters, the enzymes glutamine synthetase and glutamate synthase and PII protein, which itself regulates nitrogen metabolism. Further supporting a possible role as a cis-regulatory element in control of nitrogen metabolism, the Prochlorococcus marinus gene designed as PMT1479 was more repressed than another other gene in this organism when it was growth without a sufficient supply of nitrogen.[1][2]
It was demonstrated that glnA RNAs correspond to glutamine-binding riboswitches,[3] i.e., they sense glutamine concentrations in order to measure overall nitrogen availability, and regulate the downstream genes appropriately. The original proposal of a riboswitch function was based on the above evidence that glnA RNAs are cis-regulatory, as well as the moderate structural complexity in the three-stem junction of the glnA RNA motif that is comparable to the structures of other known riboswitches. Some glnA RNAs are located adjacent to other glnA RNAs. These "tandem arrangements" are also exhibited by glycine riboswitches and TPP riboswitches where they allow the cell to turn genes off or on within a smaller change of concentration of the riboswitch ligand. In other words, the response curve of the riboswitch better resembles a digital function.[4][5] However, such cooperative binding was not observed.[3]
A possible structural resemblance was observed between the glnA RNA motif and the Downstream-peptide motif.[1] The most apparent similarity is between the stems labeled "P1" in each motif, but other similarities were observed.[1] It was proposed that both motifs represent riboswitches involved in nitrogen metabolism. The fact that RNAs from both motifs selectively bind glutamine supports this hypothesis, but detailed structural data is not yet available. The glnA motif has an E-loop[6][7] structure (also called a bulged-G module) within it.
References
- 1 2 3 4 Weinberg Z, Wang JX, Bogue J, et al. (March 2010). "Comparative genomics reveals 104 candidate structured RNAs from bacteria, archaea, and their metagenomes". Genome Biol. 11 (3): R31. PMC 2864571
 . PMID 20230605. doi:10.1186/gb-2010-11-3-r31.
. PMID 20230605. doi:10.1186/gb-2010-11-3-r31. - ↑ Tolonen AC, Aach J, Lindell D, et al. (2006). "Global gene expression of Prochlorococcus ecotypes in response to changes in nitrogen availability". Mol. Syst. Biol. 2 (1): 53. PMC 1682016
 . PMID 17016519. doi:10.1038/msb4100087.
. PMID 17016519. doi:10.1038/msb4100087. - 1 2 Ames TD, Breaker RR (January 2011). "Bacterial aptamers that selectively bind glutamine". RNA Biol. 8 (1): 82–9. PMC 3127080
 . PMID 21282981. doi:10.4161/rna.8.1.13864.
. PMID 21282981. doi:10.4161/rna.8.1.13864. - ↑ Mandal M, Lee M, Barrick JE, et al. (October 2004). "A glycine-dependent riboswitch that uses cooperative binding to control gene expression". Science. 306 (5694): 275–9. PMID 15472076. doi:10.1126/science.1100829.
- ↑ Welz R, Breaker RR (April 2007). "Ligand binding and gene control characteristics of tandem riboswitches in Bacillus anthracis". RNA. 13 (4): 573–82. PMC 1831863
 . PMID 17307816. doi:10.1261/rna.407707.
. PMID 17307816. doi:10.1261/rna.407707. - ↑ Westhof E (2010). "The amazing world of bacterial structured RNAs". Genome Biol. 11 (3): 108. PMC 2864558
 . PMID 20236470. doi:10.1186/gb-2010-11-3-108.
. PMID 20236470. doi:10.1186/gb-2010-11-3-108. - ↑ Lee, JC (2003). Structural studies of ribosomal RNA based on cross-analysis of comparative models and three-dimensional crystal structures (PhD thesis). University of Texas.