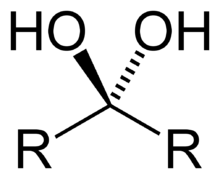
Geminal diol
A geminal diol (or gem-diol for short) is any organic compound having two hydroxyl functional groups (-OH) bound to the same carbon atom.
The simplest geminal diol is methanediol CH4O2 or H2C(OH)2. Other examples are:
- dihydroxymalonic acid HOOC-C(OH)2-COOH
- decahydroxycyclopentane (C(OH)2)5.
- (F3C)2C(OH)2, the hydrated form of hexafluoroacetone; in this case the hydration is made favorable by the electron-withdrawing trifluoromethyl groups.
- Chloral hydrate, CCl
3C(OH)
2. Again, this is stabilized by the electron-withdrawing halide atoms.
Geminal diols are a subclass of the diols, which in turn are a special class of alcohols. Geminal diols can also be viewed as an analog of hemiacetals formed by reaction of carbonyl compounds with water, instead of with an alcohol.
The two hydroxyls in a geminal diol are easily converted to a carbonyl or keto group C=O by loss of one water molecule, thus turning the diol into a ketone. Conversely, ketones tend to combine with water to form the corresponding geminal diols. The equilibrium in water solution may be shifted towards either compound; for example, the equilibrium constant for the conversion of acetone (H3C)2C=O to propane-2,2-diol (H3C)2C(OH)2 is about 10−3,[1] while that of formaldehyde H2C=O to methanediol H2C(OH)2 is 10+3,[2] and that of hexafluoroacetone (F3C)2C=O to hexafluoropropane-2,2-diol (F3C)2C(OH)2 is about 10+6. In some cases, such as decahydroxycyclopentane and dodecahydroxycyclohexane, the geminal diol is stable while the ketone is not.
See also
References
- ↑ Peter Taylor (2002), Mechanism and synthesis, Book 10 of Molecular world. Open University, Royal Society of Chemistry; ISBN 0-85404-695-X. 368 pages.
- ↑ Eric V. Anslyn, Dennis A. Dougherty (2006), Modern physical organic chemistry. University Science Books. ISBN 1-891389-31-9. 1095 pages