Gadoteric acid
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
| Formula | C16H25GdN4O8 |
| Molar mass | 558.64 g/mol |
| | |
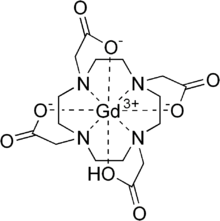
Gadoteric acid (gadoterate meglumine, trade names Artirem, Dotarem) is a macrocycle-structured gadolinium-based MRI contrast agent (GBCA). It consists of the organic acid DOTA as a chelating agent, and gadolinium (Gd3+), and is used in form of the meglumine salt (Gadoterate meglumine).[1]
It is used for imaging of blood vessels and inflamed or diseased tissue where the blood vessels become 'leaky'. It is often used when viewing intracranial lesions with abnormal vascularity or abnormalities in the blood–brain barrier. Gadoteric acid is used for MRI imaging of the brain, spine, and associated tissues for adult and pediatric (2 years of age or older) patients. The meglumine salt it takes the form of crosses the blood brain barrier of tissue with abnormal vasculature, highlighting the affected area with MRI. Gadoterate does not cross the intact blood-brain barrier, so it does not affect or enhance normal brain tissue in imaging.[2] Dotarem is administered through an intravenous bolus injection, either manually or through a power injection.[1]
The most common adverse effects (>0.2%) in clinical studies were nausea, headache, injection site pain, injection site coldness, and burning sensation. Drugs with gadolinium-based contrasting agents can increase the risk of nephrogenic systemic fibrosis (NSF) for those with impaired elimination of the drug. Those most at risk for NSF include patients with chronic or severe kidney disease and acute kidney injury.[1][3]
The paramagnetic property of gadoteric acid reduces the T1 relaxation time (and to some extent the T2 and T2* relaxation times) in MRI, which is the source of its clinical utility. Because it has magnetic properties, gadoteric acid develops a magnetic moment when put under a magnetic field, which increases the signal intensity (brightness) of tissues during MRI imaging.[2]
The drug, under the brand Dotarem, was brought to market by Guerbet.[4] As of 2013 gadoteric acid was approved in around 70 countries.[5][6] It was launched in the French market in 1989 and was FDA approved in the US in March, 2013.[4] Dotarem is the seventh FDA approved GBCA for use in central nervous system (CNS) MRI. In 2017, GE Healthcare launched a gadoteric acid (as gadoterate meglumine) under the trade name Clariscan[7] Other FDA approved GBCAs for similar purposes include Magnevist (1988), Prohance (1992), Omniscan (1993), Optimark (1999), Multihance (2004) and Gadavist (2011).[8]
References
- 1 2 3 "US gadoterate meglumine label" (PDF). FDA. March 2013.
- 1 2 DrugBank, ed. (2016-08-22). "Gadoteric acid". DrugBank.
- ↑ Todd, DJ; Kay, J (2016). "Gadolinium-Induced Fibrosis.". Annual Review of Medicine. 67: 273–91. PMID 26768242.
- 1 2 Hollmer, Mark (January 6, 2014). "Dotarem: A safe(r) gadolinium-based contrast imaging agent". FierceBiotech.
- ↑ "Gadoteric Acid international brands". Drugs.com. Retrieved 7 March 2017.
- ↑ Guerbet LLC (14 February 2013). "Advisory Committee Briefing Document for NDA 204-781". FDA.
- ↑ http://www.mhra.gov.uk/home/groups/spcpil/documents/spcpil/con1487912585095.pdf
- ↑ "Press Announcements - FDA approves Dotarem, a new magnetic resonance imaging agent". www.fda.gov. Retrieved 2016-11-14.