Gadolinium(III) nitrate
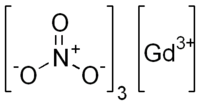 | |
| Identifiers | |
|---|---|
| 3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.030.385 |
| PubChem CID |
|
| |
| |
| Properties | |
| Gd(NO3)3 | |
| Molar mass | 343.26 g/mol |
| Appearance | White crystalline solid |
| Density | 2.3 g/cm3 |
| Melting point | 91 °C (196 °F; 364 K) |
| Soluble | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Gadolinium(III) nitrate is an inorganic compound of gadolinium. It is used as a water-soluble neutron poison in nuclear reactors.[1] Gadolinium nitrate, like all nitrates, is an oxidizing agent.
Use
Gadolinium nitrate was used at the Savannah River Site heavy water nuclear reactors and has to be separated from the heavy water for storage or reuse.[2][3] The Canadian CANDU reactor, a pressurized heavy water reactor, also uses gadolinium nitrate as a water-soluble neutron poison in heavy water.
Gadolinium nitrate is also used as a raw material in the production of other gadolinium compounds, for production of specialty glasses and ceramics and as a phosphor.
References
- ↑ DOE Fundamentals Handbook: Nuclear Physics and Reactor Theory (PDF). U.S. Department of Energy. January 1993. p. 31. Retrieved 2007-09-26.
- ↑ E. Wilde; C. Berry. "Novel Method for Removing Gadolinium from Used Heavy Water Reactor Moderator".
- ↑ E.W. Wilde; M.B. Goli; C.J. Berry; J.W. Santo Domingo; H.L. Martin. "Novel Method for Removing Gadolinium from Used Heavy Water Reactor Moderator" (PDF).
| Salts and covalent derivatives of the Nitrate ion | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HNO3 | He | ||||||||||||||||||
| LiNO3 | Be(NO3)2 | B(NO3)4− | C | N | O | FNO3 | Ne | ||||||||||||
| NaNO3 | Mg(NO3)2 | Al(NO3)3 | Si | P | S | ClONO2 | Ar | ||||||||||||
| KNO3 | Ca(NO3)2 | Sc(NO3)3 | Ti(NO3)4 | VO(NO3)3 | Cr(NO3)3 | Mn(NO3)2 | Fe(NO3)3, Fe(NO3)2 |
Co(NO3)2, Co(NO3)3 |
Ni(NO3)2 | Cu(NO3)2 | Zn(NO3)2 | Ga(NO3)3 | Ge | As | Se | Br | Kr | ||
| RbNO3 | Sr(NO3)2 | Y | Zr(NO3)4 | Nb | Mo | Tc | Ru | Rh | Pd(NO3)2 | AgNO3 | Cd(NO3)2 | In | Sn | Sb(NO3)3 | Te | I | Xe(NO3)2 | ||
| CsNO3 | Ba(NO3)2 | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg2(NO3)2, Hg(NO3)2 |
Tl(NO3)3, TlNO3 |
Pb(NO3)2 | Bi(NO3)3 BiO(NO3) |
Po | At | Rn | |||
| FrNO3 | Ra(NO3)2 | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |||
| ↓ | |||||||||||||||||||
| La(NO3)3 | Ce(NO3)3, Ce(NO3)4 |
Pr | Nd | Pm | Sm | Eu | Gd(NO3)3 | Tb | Dy | Ho | Er | Tm | Yb | Lu | |||||
| Ac(NO3)3 | Th(NO3)4 | Pa | UO2(NO3)2 | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | |||||
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.