GYKI-52,466
 | |
 | |
| Names | |
|---|---|
| IUPAC name
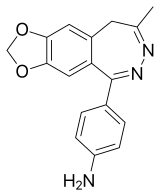
4-(8-methyl-9H-[1,3]dioxolo[4,5-h][2,3]benzodiazepin-5-yl)aniline | |
| Identifiers | |
| |
| 3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.162.378 |
| KEGG | |
| PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C17H15N3O2 | |
| Molar mass | 293.32 g/mol |
| Appearance | Yellow solid (HCl salt) |
| Density | 1.393 g/cm3 |
| >10 mg/mL (HCl salt) | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
GYKI-52466 is a 2,3-benzodiazepine that acts as an ionotropic glutamate receptor antagonist, which is a non-competitive AMPA receptor antagonist (IC50 values are 10-20, ~ 450 and >> 50 μM for AMPA- , kainate- and NMDA-induced responses respectively), orally-active anticonvulsant, and skeletal muscle relaxant. Unlike conventional 1,4-benzodiazepines, GYKI-52466 and related 2,3-benzodiazepines do not act on GABAA receptors. Like other AMPA receptor antagonists, GYKI-52466 has anticonvulsant and neuroprotective properties.
See also
- GYKI-52895, another 2,3-benzodiazepine with other than GABAergic function
- Tifluadom
- Lufuradom
References
- ↑ Donevan, S.D., Rogawski, M.A., Allosteric regulation of alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionate receptors by thiocyanate and cyclothiazide at a common modulatory site distinct from that of 2,3-benzodiazepines. Neuroscience 87, 615, (1998)
- ↑ Donevan, S.D., Rogawski, M.A., GYKI 52466, a 2,3-benzodiazepine is a highly selective, non-competitive antagonist of AMPA/kainate receptor responses. Neuron 10, 51, (1993)
- ↑ Wilding, T.J., Huettner, J.E., Differential antagonism of alpha-amino-3-hydroxy-5-methyl-4- isoxazolepropionic acid-preferring and kainate-preferring receptors by 2,3-benzodiazepines. Mol. Pharmacol. 47, 582, (1995)
- ↑ Tarnawa et al. (1989) Electrophysiological studies with a 2,3-benzodiazepine muscle relaxant: GYKI 52466. Eur. J.Pharmacol. 167 193
- ↑ Paternain et al. (1995) Selective antagonism of AMPA receptors unmasks kainate receptor-mediated responses in hippocampal neurons. Neuron 14 185.
- ↑ Rzeski et al. (2001) Glutamate antagonists limit tumor growth. Proc. Natl.Acad. Sci.USA 98 6372.
- ↑ Szabados et al. (2001) Comparison of anticonvulsive and acute neuroprotective activity of three 2,3-benzodiazepine compounds, GYKI 52466, GYKI 53405, and GYKI 53655. Brain Res. Bull. 55 387.
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.