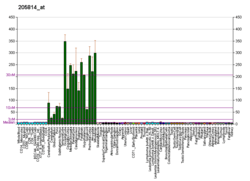
Metabotropic glutamate receptor 3
Metabotropic glutamate receptor 3 is a protein that in humans is encoded by the GRM3 gene.[5][6]
Function
L-glutamate is the major excitatory neurotransmitter in the central nervous system and activates both ionotropic and metabotropic glutamate receptors. Glutamatergic neurotransmission is involved in most aspects of normal brain function and can be perturbed in many neuropathologic conditions. The metabotropic glutamate receptors are a family of G protein-coupled receptors, that have been divided into 3 groups on the basis of sequence homology, putative signal transduction mechanisms, and pharmacologic properties. Group I includes GRM1 and GRM5 and these receptors have been shown to activate phospholipase C. Group II includes GRM2 and GRM3 while Group III includes GRM4, GRM6, GRM7 and GRM8. Group II and III receptors are linked to the inhibition of the cyclic AMP cascade but differ in their agonist selectivities.[6]
Clinical significance
The mGluR3 receptor encoded by the GRM3 gene has been found to be associated with bipolar affective disorder.[7] A mutation in the Kozak sequence in the 1st exon of the GRM3 gene was shown to change translation and transcription of cloned GRM3 gene constructs and was significantly associated with bipolar disorder with an odds ratio of 4.4.[7] Subsequently, a marker in GRM3 was implicated in a large genome-wide association study of schizophrenia with statistical significance of p<10−9.[8] A follow-up study of the Kozak sequence variant showed that it was associated with increased risk of bipolar disorder, schizophrenia and alcoholism.[9] Thus GRM3 is likely to contribute to the genetic susceptibility of a variety of mental disorders. The mGluR3 receptor encoded by GRM3 is targetable by several drugs that have been used in previous trials of schizophrenia and anxiety disorder. The agonist, antagonist and allosteric modulator drugs of mGluR3 can now be explored as new treatments for mental illness. This might become the first example of personalised medicine based on genetics for psychiatric disorders.[7] Other scientific evidence has been published which shows that the well established anti-manic drug lithium carbonate also changes GRM3 gene expression in the mouse brain after treatment with lithium carbonate.[10]
Ligands
mGluR3 modulators that are significantly selective over the isoform mGluR2 are known since 2013.
Agonists
- with a bicyclo[3.1.0]hexane skeleton
- (R)-2-amino-4-(4-hydroxy[1,2,5]thiadiazol-3-yl)butyric acid[16]
Antagonists
- CECXG – 38x selectivity for mGlu3 over mGlu2
- LY-341,495 and its 1-fluoro analog:[17] potent orthosteric antagonists
- MGS-0039,[18] HYDIA[19] (both with bicyclo[3.1.0]hexane skeleton)
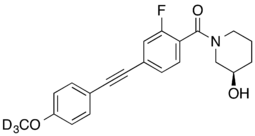
Allosteric modulators
- D3-ML337: selective NAM, IC50 = 450 nM for mGluR3, IC50 >30μM for mGluR2[20]
- MNI-137:[21] inhibitior
- compound 7p:[22] non-competitive antagonist (presumably allosteric inhibitor)
Interactions
Metabotropic glutamate receptor 3 has been shown to interact with:
See also
References
- 1 2 3 GRCh38: Ensembl release 89: ENSG00000198822 - Ensembl, May 2017
- 1 2 3 GRCm38: Ensembl release 89: ENSMUSG00000003974 - Ensembl, May 2017
- ↑ "Human PubMed Reference:".
- ↑ "Mouse PubMed Reference:".
- ↑ Scherer SW, Duvoisin RM, Kuhn R, Heng HH, Belloni E, Tsui LC (Mar 1997). "Localization of two metabotropic glutamate receptor genes, GRM3 and GRM8, to human chromosome 7q". Genomics. 31 (2): 230–3. PMID 8824806. doi:10.1006/geno.1996.0036.
- 1 2 "Entrez Gene: GRM3 glutamate receptor, metabotropic 3".
- 1 2 3 Kandaswamy R, McQuillin A, Sharp SI, Fiorentino A, Anjorin A, Blizard RA, Curtis D, Gurling HM (2013). "Genetic association, mutation screening, and functional analysis of a Kozak sequence variant in the metabotropic glutamate receptor 3 gene in bipolar disorder". JAMA Psychiatry. 70 (6): 591–8. PMID 23575746. doi:10.1001/jamapsychiatry.2013.38.
- ↑ Schizophrenia Working Group of the Psychiatric Genomics Consortium (2014). "Biological insights from 108 schizophrenia-associated genetic loci.". Nature. 511 (7510): 421–7. PMC 4112379
 . PMID 25056061. doi:10.1038/nature13595.
. PMID 25056061. doi:10.1038/nature13595. - ↑ O'Brien NL, Way MJ, Kandaswamy R, Fiorentino A, Sharp SI, Quadri G, Alex J, Anjorin A, Ball D, Cherian R, Dar K, Gormez A, Guerrini I, Heydtmann M, Hillman A, Lankappa S, Lydall G, O'Kane A, Patel S, Quested D, Smith I, Thomson AD, Bass NJ, Morgan MY, Curtis D, McQuillin A (2014). "The functional GRM3 Kozak sequence variant rs148754219 affects the risk of schizophrenia and alcohol dependence as well as bipolar disorder". Psychiatr. Genet. 24: 277–278. PMID 25046171. doi:10.1097/YPG.0000000000000050.
- ↑ McQuillin A, Rizig M, Gurling HM (2007). "A microarray gene expression study of the molecular pharmacology of lithium carbonate on mouse brain mRNA to understand the neurobiology of mood stabilization and treatment of bipolar affective disorder". Pharmacogenet. Genomics. 17 (8): 605–17. PMID 17622937. doi:10.1097/FPC.0b013e328011b5b2.
- ↑ Nakazato A, Kumagai T, Sakagami K, Yoshikawa R, Suzuki Y, Chaki S, Ito H, Taguchi T, Nakanishi S, Okuyama S (2000). "Synthesis, SARs, and pharmacological characterization of 2-amino-3 or 6-fluorobicyclo[3.1.0]hexane-2,6-dicarboxylic acid derivatives as potent, selective, and orally active group II metabotropic glutamate receptor agonists". Journal of Medicinal Chemistry. 43 (25): 4893–909. PMID 11123999. doi:10.1021/jm000346k.
- ↑ Monn JA, Massey SM, Valli MJ, Henry SS, Stephenson GA, Bures M, Hérin M, Catlow J, Giera D, Wright RA, Johnson BG, Andis SL, Kingston A, Schoepp DD (2007). "Synthesis and metabotropic glutamate receptor activity of S-oxidized variants of (−)-4-amino-2-thiabicyclo-[3.1.0]hexane-4,6-dicarboxylate: identification of potent, selective, and orally bioavailable agonists for mGlu2/3 receptors". Journal of Medicinal Chemistry. 50 (2): 233–40. PMID 17228865. doi:10.1021/jm060917u.
- ↑ Monn JA, Valli MJ, Massey SM, Hansen MM, Kress TJ, Wepsiec JP, Harkness AR, Grutsch JL, Wright RA, Johnson BG, Andis SL, Kingston A, Tomlinson R, Lewis R, Griffey KR, Tizzano JP, Schoepp DD (1999). "Synthesis, pharmacological characterization, and molecular modeling of heterobicyclic amino acids related to (+)-2-aminobicyclo[3.1.0] hexane-2,6-dicarboxylic acid (LY354740): identification of two new potent, selective, and systemically active agonists for group II metabotropic glutamate receptors". Journal of Medicinal Chemistry. 42 (6): 1027–40. PMID 10090786. doi:10.1021/jm980616n.
- ↑ Monn JA, Valli MJ, Massey SM, Wright RA, Salhoff CR, Johnson BG, Howe T, Alt CA, Rhodes GA, Robey RL, Griffey KR, Tizzano JP, Kallman MJ, Helton DR, Schoepp DD (1997). "Design, synthesis, and pharmacological characterization of (+)-2-aminobicyclo[3.1.0]hexane-2,6-dicarboxylic acid (LY354740): a potent, selective, and orally active group 2 metabotropic glutamate receptor agonist possessing anticonvulsant and anxiolytic properties". Journal of Medicinal Chemistry. 40 (4): 528–37. PMID 9046344. doi:10.1021/jm9606756.
- ↑ Dominguez C, Prieto L, Valli MJ, Massey SM, Bures M, Wright RA, Johnson BG, Andis SL, Kingston A, Schoepp DD, Monn JA (2005). "Methyl substitution of 2-aminobicyclo[3.1.0]hexane 2,6-dicarboxylate (LY354740) determines functional activity at metabotropic glutamate receptors: identification of a subtype selective mGlu2 receptor agonist". Journal of Medicinal Chemistry. 48 (10): 3605–12. PMID 15887967. doi:10.1021/jm040222y.
- ↑ Clausen RP, Bräuner-Osborne H, Greenwood JR, Hermit MB, Stensbøl TB, Nielsen B, Krogsgaard-Larsen P (2002). "Selective agonists at group II metabotropic glutamate receptors: synthesis, stereochemistry, and molecular pharmacology of (S)- and (R)-2-amino-4-(4-hydroxy[1,2,5]thiadiazol-3-yl)butyric acid". Journal of Medicinal Chemistry. 45 (19): 4240–5. PMID 12213064. doi:10.1021/jm020122x.
- ↑ Sakagami K, Yasuhara A, Chaki S, Yoshikawa R, Kawakita Y, Saito A, Taguchi T, Nakazato A (2008). "Synthesis, in vitro pharmacology, and pharmacokinetic profiles of 2-[1-amino-1-carboxy-2-(9H-xanthen-9-yl)-ethyl]-1-fluorocyclopropanecarboxylic acid and its 6-heptyl ester, a potent mGluR2 antagonist". Bioorg. Med. Chem. 16 (8): 4359–66. PMID 18348906. doi:10.1016/j.bmc.2008.02.066.
- ↑ a) Nakazato A, Sakagami K, Yasuhara A, Ohta H, Yoshikawa R, Itoh M, Nakamura M, Chaki S (2004). "Synthesis, in vitro pharmacology, structure-activity relationships, and pharmacokinetics of 3-alkoxy-2-amino-6-fluorobicyclo[3.1.0]hexane-2,6-dicarboxylic acid derivatives as potent and selective group II metabotropic glutamate receptor antagonists". Journal of Medicinal Chemistry. 47 (18): 4570–87. PMID 15317467. doi:10.1021/jm0400294.,
b) Yasuhara A, Nakamura M, Sakagami K, Shimazaki T, Yoshikawa R, Chaki S, Ohta H, Nakazato A (2006). "Prodrugs of 3-(3,4-dichlorobenzyloxy)-2-amino-6-fluorobicyclo[3.1.0]hexane-2,6-dicarboxylic acid (MGS0039): a potent and orally active group II mGluR antagonist with antidepressant-like potential". Bioorg. Med. Chem. 14 (12): 4193–207. PMID 16487713. doi:10.1016/j.bmc.2006.01.060.,
c) Yasuhara A, Sakagami K, Yoshikawa R, Chaki S, Nakamura M, Nakazato A (2006). "Synthesis, in vitro pharmacology, and structure-activity relationships of 2-aminobicyclo[3.1.0]hexane-2,6-dicarboxylic acid derivatives as mGluR2 antagonists". Bioorg. Med. Chem. 14 (10): 3405–20. PMID 16431115. doi:10.1016/j.bmc.2005.12.061. - ↑ Woltering TJ, Adam G, Huguenin P, Wichmann J, Kolczewski S, Gatti S, Bourson A, Kew JN, Richards G, Kemp JA, Mutel V, Knoflach F (2008). "Asymmetric synthesis and receptor pharmacology of the group II mGlu receptor ligand (1S,2R,3R,5R,6S)-2-amino-3-hydroxy-bicyclo[3.1.0]hexane-2,6-dicarboxylic acid-HYDIA". ChemMedChem. 3 (2): 323–35. PMID 18058780. doi:10.1002/cmdc.200700226.
- ↑ Wenthur CJ, Morrison R, Felts AS, Smith KA, Engers JL, Byers FW, Daniels JS, Emmitte KA, Conn PJ, Lindsley CW (2013). "Discovery of (R)-(2-fluoro-4-((-4-methoxyphenyl)ethynyl)phenyl) (3-hydroxypiperidin-1-yl)methanone (ML337), an mGlu3 selective and CNS penetrant negative allosteric modulator (NAM)". J. Med. Chem. 56 (12): 5208–12. PMC 3769689
 . PMID 23718281. doi:10.1021/jm400439t.
. PMID 23718281. doi:10.1021/jm400439t. - ↑ Hemstapat K, Da Costa H, Nong Y, Brady AE, Luo Q, Niswender CM, Tamagnan GD, Conn PJ (2007). "A novel family of potent negative allosteric modulators of group II metabotropic glutamate receptors". J. Pharmacol. Exp. Ther. 322 (1): 254–64. PMID 17416742. doi:10.1124/jpet.106.117093.
- ↑ Woltering TJ, Wichmann J, Goetschi E, Adam G, Kew JN, Knoflach F, Ballard TM, Huwyler J, Mutel V, Gatti S (2008). "Synthesis and characterization of 1,3-dihydro-benzo[b][1,4]diazepin-2-one derivatives: Part 3. New potent non-competitive metabotropic glutamate receptor 2/3 antagonists". Bioorg. Med. Chem. Lett. 18 (8): 2725–9. PMID 18374569. doi:10.1016/j.bmcl.2008.02.076.
- 1 2 Hirbec H, Perestenko O, Nishimune A, Meyer G, Nakanishi S, Henley JM, Dev KK (May 2002). "The PDZ proteins PICK1, GRIP, and syntenin bind multiple glutamate receptor subtypes. Analysis of PDZ binding motifs". J. Biol. Chem. 277 (18): 15221–4. PMID 11891216. doi:10.1074/jbc.C200112200.
- ↑ Flajolet M, Rakhilin S, Wang H, Starkova N, Nuangchamnong N, Nairn AC, Greengard P (Dec 2003). "Protein phosphatase 2C binds selectively to and dephosphorylates metabotropic glutamate receptor 3". Proc. Natl. Acad. Sci. U.S.A. 100 (26): 16006–11. PMC 307683
 . PMID 14663150. doi:10.1073/pnas.2136600100.
. PMID 14663150. doi:10.1073/pnas.2136600100.
Further reading
- Makoff A, Volpe F, Lelchuk R, Harrington K, Emson P (1997). "Molecular characterization and localization of human metabotropic glutamate receptor type 3.". Brain Res. Mol. Brain Res. 40 (1): 55–63. PMID 8840013. doi:10.1016/0169-328X(96)00037-X.
- Emile L, Mercken L, Apiou F, Pradier L, Bock MD, Menager J, Clot J, Doble A, Blanchard JC (1997). "Molecular cloning, functional expression, pharmacological characterization and chromosomal localization of the human metabotropic glutamate receptor type 3.". Neuropharmacology. 35 (5): 523–30. PMID 8887960. doi:10.1016/0028-3908(96)84622-3.
- Corti C, Sala CF, Yang F, Corsi M, Xuereb JH, Ferraguti F (2001). "Genomic organization of the human metabotropic glutamate receptor subtype 3.". J. Neurogenet. 14 (4): 207–25, 271. PMID 11342382. doi:10.3109/01677060009084499.
- Corti C, Xuereb JH, Corsi M, Ferraguti F (2001). "Identification and characterization of the promoter region of the GRM3 gene.". Biochem. Biophys. Res. Commun. 286 (2): 381–7. PMID 11500049. doi:10.1006/bbrc.2001.5391.
- Tomiyama M, Kimura T, Maeda T, Tanaka H, Furusawa K, Kurahashi K, Matsunaga M (2001). "Expression of metabotropic glutamate receptor mRNAs in the human spinal cord: implications for selective vulnerability of spinal motor neurons in amyotrophic lateral sclerosis.". J. Neurol. Sci. 189 (1-2): 65–9. PMID 11535235. doi:10.1016/S0022-510X(01)00561-5.
- Rosemond E, Peltekova V, Naples M, Thøgersen H, Hampson DR (2002). "Molecular determinants of high affinity binding to group III metabotropic glutamate receptors.". J. Biol. Chem. 277 (9): 7333–40. PMID 11744707. doi:10.1074/jbc.M110476200.
- Martí SB, Cichon S, Propping P, Nöthen M (2002). "Metabotropic glutamate receptor 3 (GRM3) gene variation is not associated with schizophrenia or bipolar affective disorder in the German population.". American Journal of Medical Genetics. 114 (1): 46–50. PMID 11840505. doi:10.1002/ajmg.1624.
- Kitano J, Kimura K, Yamazaki Y, Soda T, Shigemoto R, Nakajima Y, Nakanishi S (2002). "Tamalin, a PDZ domain-containing protein, links a protein complex formation of group 1 metabotropic glutamate receptors and the guanine nucleotide exchange factor cytohesins.". J. Neurosci. 22 (4): 1280–9. PMID 11850456.
- Hirbec H, Perestenko O, Nishimune A, Meyer G, Nakanishi S, Henley JM, Dev KK (2002). "The PDZ proteins PICK1, GRIP, and syntenin bind multiple glutamate receptor subtypes. Analysis of PDZ binding motifs.". J. Biol. Chem. 277 (18): 15221–4. PMID 11891216. doi:10.1074/jbc.C200112200.
- Fujii Y, Shibata H, Kikuta R, Makino C, Tani A, Hirata N, Shibata A, Ninomiya H, Tashiro N, Fukumaki Y (2004). "Positive associations of polymorphisms in the metabotropic glutamate receptor type 3 gene (GRM3) with schizophrenia.". Psychiatr. Genet. 13 (2): 71–6. PMID 12782962. doi:10.1097/01.ypg.0000056682.82896.b0.
- Aronica E, Gorter JA, Ijlst-Keizers H, Rozemuller AJ, Yankaya B, Leenstra S, Troost D (2003). "Expression and functional role of mGluR3 and mGluR5 in human astrocytes and glioma cells: opposite regulation of glutamate transporter proteins.". Eur. J. Neurosci. 17 (10): 2106–18. PMID 12786977. doi:10.1046/j.1460-9568.2003.02657.x.
- Flajolet M, Rakhilin S, Wang H, Starkova N, Nuangchamnong N, Nairn AC, Greengard P (2004). "Protein phosphatase 2C binds selectively to and dephosphorylates metabotropic glutamate receptor 3.". Proc. Natl. Acad. Sci. U.S.A. 100 (26): 16006–11. PMC 307683
 . PMID 14663150. doi:10.1073/pnas.2136600100.
. PMID 14663150. doi:10.1073/pnas.2136600100. - Yao Y, Koo JC, Wells JW, Hampson DR (2004). "Expression of a truncated secreted form of the mGluR3 subtype of metabotropic glutamate receptor.". Biochem. Biophys. Res. Commun. 319 (2): 622–8. PMID 15178451. doi:10.1016/j.bbrc.2004.05.032.
- Tang FR, Chia SC, Chen PM, Gao H, Lee WL, Yeo TS, Burgunder JM, Probst A, Sim MK, Ling EA (2004). "Metabotropic glutamate receptor 2/3 in the hippocampus of patients with mesial temporal lobe epilepsy, and of rats and mice after pilocarpine-induced status epilepticus.". Epilepsy Res. 59 (2-3): 167–80. PMID 15246118. doi:10.1016/j.eplepsyres.2004.04.002.
- Egan MF, Straub RE, Goldberg TE, Yakub I, Callicott JH, Hariri AR, Mattay VS, Bertolino A, Hyde TM, Shannon-Weickert C, Akil M, Crook J, Vakkalanka RK, Balkissoon R, Gibbs RA, Kleinman JE, Weinberger DR (2004). "Variation in GRM3 affects cognition, prefrontal glutamate, and risk for schizophrenia.". Proc. Natl. Acad. Sci. U.S.A. 101 (34): 12604–9. PMC 515104
 . PMID 15310849. doi:10.1073/pnas.0405077101.
. PMID 15310849. doi:10.1073/pnas.0405077101.
External links
- "Metabotropic Glutamate Receptors: mGlu3". IUPHAR Database of Receptors and Ion Channels. International Union of Basic and Clinical Pharmacology.
This article incorporates text from the United States National Library of Medicine, which is in the public domain.