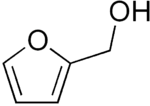
Furfuryl alcohol
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
(Furan-2-yl)methanol | |
| Other names
Furan-2-ylmethanol Furfuryl alcohol 2-Furanmethanol 2-Furancarbinol 2-(Hydroxymethyl)furan | |
| Identifiers | |
| 3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.002.388 |
| PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C5H6O2 | |
| Molar mass | 98.10 g/mol |
| Appearance | colorless to amber liquid[2] |
| Odor | burning odor[2] |
| Density | 1.128 g/cm3 |
| Melting point | −29 °C (−20 °F; 244 K) |
| Boiling point | 170 °C (338 °F; 443 K) |
| miscible | |
| Hazards | |
| Safety data sheet | External MSDS |
| NFPA 704 | |
| Flash point | 65 °C; 149 °F; 338 K [2] |
| Explosive limits | 1.8% - 16.3%[2] |
| Lethal dose or concentration (LD, LC): | |
| LC50 (median concentration) |
397 ppm (mouse, 6 hr) 85 ppm (rat, 6 hr) 592 ppm (rat, 1 hr)[3] |
| LCLo (lowest published) |
597 ppm (mouse, 6 hr)[3] |
| US health exposure limits (NIOSH): | |
| PEL (Permissible) |
TWA 50 ppm (200 mg/m3)[2] |
| REL (Recommended) |
TWA 10 ppm (40 mg/m3) ST 15 ppm (60 mg/m3) [skin][2] |
| IDLH (Immediate danger) |
75 ppm[2] |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Furfuryl alcohol, also called 2-furylmethanol or 2-furancarbinol, is an organic compound containing a furan substituted with a hydroxymethyl group. It is a clear colorless liquid when pure, but becomes amber colored upon prolonged standing. It possesses a faint odor of burning and a bitter taste. It is miscible with but unstable in water. It is soluble in common organic solvents. Upon treatment with acids, heat and/or catalysts, furfuryl alcohol can be made to polymerize into a resin, poly(furfuryl alcohol).
Synthesis
Furfuryl alcohol is manufactured industrially from furfural; which is itself typically produced from waste bio-mass such as corncobs or sugar cane bagasse. As such furfuryl alcohol may be considered a green chemical. It is produced either by the direct reduction of furfural, or by its disproportionation via the Cannizaro reaction in an aqueous NaOH solution; this latter route also produces furoic acid.[4]
Applications
Plastics
The primary use of furfuryl alcohol is as a monomer for the synthesis of furan resins. These are used in thermoset polymer matrix composites, cements, adhesives, coatings and casting/foundry resins. Synthesis involves acid-catalyzed polycondensation, usually giving a black cross-linked product. A simplified example is shown below but the actual reaction mechanism is known to be exceedingly complex.[5]

Other uses
Furfuryl alcohol has been used in rocketry as a fuel which ignites hypergolically (immediately and energetically in contact) with white fuming nitric acid or red fuming nitric acid oxidizer.[6] The use of hypergolics avoids the need for an igniter. In late 2012, Spectra, a concept liquid rocket engine using white fuming nitric acid as the oxidizer to furfuryl alcohol fuel was static tested by Copenhagen Suborbitals.[7][8]
Because of its low molecular weight, furfuryl alcohol can impregnate the cells of wood, where it can be polymerized and bonded with the wood by heat, radiation, and/or catalysts or additional reactants. The treated wood has improved moisture-dimensional stability, hardness, and decay and insect resistance; catalysts can include zinc chloride, citric or formic acid, or borates.[9][10]
See also
- Furfurylamine - corresponding amine
- 2-Furonitrile - corresponding nitrile
- Furan-2-ylmethanethiol - corresponding thiol
- 2-Furoic acid - corresponding carboxylic acid
References
- ↑ Merck Index, 11th Edition, 4215.
- 1 2 3 4 5 6 7 "NIOSH Pocket Guide to Chemical Hazards #0298". National Institute for Occupational Safety and Health (NIOSH).
- 1 2 "Furfuryl alcohol". Immediately Dangerous to Life and Health. National Institute for Occupational Safety and Health (NIOSH).
- ↑ Mariscal, R.; Maireles-Torres, P.; Ojeda, M.; Sádaba, I.; López Granados, M. (2016). "Furfural: a renewable and versatile platform molecule for the synthesis of chemicals and fuels". Energy Environ. Sci. 9 (4): 1144–1189. ISSN 1754-5692. doi:10.1039/C5EE02666K.
- ↑ Choura, Mekki; Belgacem, Naceur M.; Gandini, Alessandro (January 1996). "Acid-Catalyzed Polycondensation of Furfuryl Alcohol: Mechanisms of Chromophore Formation and Cross-Linking". Macromolecules. 29 (11): 3839–3850. doi:10.1021/ma951522f.
- ↑ MUNJAL, N. L. (May 1970). "Ignition catalysts for furfuryl alcohol - Red fuming nitric acid bipropellant". AIAA Journal. 8 (5): 980–981. doi:10.2514/3.5816.
- ↑ Madsen, Peter. "Spectra-testen". Retrieved September 10, 2012.
- ↑ http://copenhagensuborbitals.com/public/spectra.pdf The Spectra engine test report pdf
- ↑ Alfred J., Stamm (1977). "Chapter 9". Wood Technology: Chemical Aspects. Washington: American Chemical Society. pp. 141–149. ISBN 9780841203730.
- ↑ Baysal, Ergun; Ozaki, S.Kiyoka; Yalinkilic, MustafaKemal (21 August 2004). "Dimensional stabilization of wood treated with furfuryl alcohol catalysed by borates". Wood Science and Technology. doi:10.1007/s00226-004-0248-2.
External links
- Health hazards
- CDC - NIOSH Pocket Guide to Chemical Hazards
- Literature: K.J. Zeitsch,The Chemistry and Technology of Furfural and its Many By-Products, Elsevier, 2000
