Fostamatinib
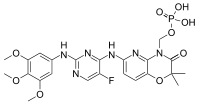 Fostamatinib | |
 Fostamatinib disodium | |
| Names | |
|---|---|
| IUPAC name
[6-({5-Fluoro-2-[(3,4,5-trimethoxyphenyl)amino]pyrimidin-4-yl}amino)-2,2-dimethyl-3-oxo-2,3-dihydro-4H-pyrido[3,2-b][1,4]oxazin-4-yl]methyl dihydrogen phosphate | |
| Other names
Tamatinib fosdium; R-788; NSC-745942; R-935788 | |
| Identifiers | |
| |
| 3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.125.771 |
| PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C23H26FN6O9P | |
| Molar mass | 580.47 g·mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Fostamatinib is an experimental drug candidate for the treatment of a variety of diseases, originally developed by Rigel Pharmaceuticals. The drug is administered orally as a disodium salt, and is a prodrug of the active compound tamatinib (R-406),[1] which is an inhibitor of the enzyme spleen tyrosine kinase (Syk),[2] hence it is an syk inhibitor.
Syk is a protein tyrosine kinase associated with various inflammatory cells, including macrophages, which are presumed to be the cells responsible for ITP platelet clearance.[3] When FcγRs I, IIA, and IIIA bind to their ligands, the receptor complex becomes activated and triggers the phosphorylation of the immunoreceptor-activating motifs (ITAMs). This leads to various genes becoming activated, which causes a cytoskeletal rearrangement that mediates phagocytosis in cells of the monocyte/macrophage lineage. Because Syk plays an important role in FcγR-mediated signal transduction and inflammatory propagation, it is considered a good target for the inhibition of various autoimmune conditions, including rheumatoid arthritis and lymphoma.
Clinical trials
Fostamatinib has been in clinical trials for rheumatoid arthritis, autoimmune thrombocytopenia, autoimmune hemolytic anemia, IgA nephropathy, and lymphoma.[1][4]
The investigation of fostamatinib began with studies involving the treatment of mouse models with cytopenia. Mice were used to measure the effectiveness of R788, a small molecule prodrug of the biologically active R406, a Syk inhibitor. In animal models, treatment with R406/R788 was shown to be safe and effective in reducing inflammation and joint damage in immune-mediated rheumatoid arthritis. The models responded favorably to treatment so the study progressed to Phase 2 trials involving humans. Human studies have shown that R788 has good oral bioavailability, biologic activity, is well tolerated, and does not exhibit collagen or ADP-induced platelet aggregation. In NCT00706342, 16 adults with chronic ITP were entered into an open-label, single-arm cohort dose-escalation trials beginning with 75 mg and rising to 175 mg twice a day. The dose was increased until a persistent response was evident, toxicity was reached, or 175 mg twice a day was met. 8 patients achieved persistent responses with platelet counts greater than 50,000 mm3/L on more than 67% of their visits. 3 of these patients had not persistently responded to thrombopoietic agents. 4 others had nonsustained responses. Mean peak platelet count exceeded 100,000 mm3/L in these 12 patients. Toxicity was evidenced primarily in GI-related side effects, notable diarrhea, urgency, and vomiting. 2 patients developed transaminitis.[5]
Rheumatoid Arthritis
A phase II study of rheumatoid arthritis patients failing to respond to a biologic agent showed little efficacy as compared to placebo, but the drug was well tolerated. In patients with high inflammatory burden, measured by levels of C-reactive protein, ACR20 was achieved by a significantly higher portion of those in the fostamatinib group (42%) versus the placebo group (26%).[6]
Autoimmune Thrombocytopenia (ITP)
Immune thrombocytopenic purpura (ITP) is an autoimmune disease where the immune system attacks and destroys platelets in the blood, causing abnormally low platelet counts. It is characterized by the antibody-mediated destruction of platelets. Patients with ITP have accelerated clearance of circulating IgG-coated platelets via Fcγ receptor-bearing macrophages in the spleen and liver, leading to different levels of thrombocytopenia and variable degrees of mucocutaneous bleeding.[7] Recent studies of ITP pathophysiology suggest decreased platelet production may also be an important component of the thrombocytopenia. Many patients exhibit responses to established therapies, including corticosteroids, IV immunoglobulin, anti-D, splenectomy, and rituximab. However, there are a significant minority of patients who retain persistently low platelet counts despite treatment. These patients are consistently at risk of intracranial hemorrhage and other bleeding complications. Several thrombopoiesis-stimulating therapies including eltrombopag and AMG 531 are being investigated to help combat low platelet counts in ITP patients. Rigel reported results from two Phase III clinical trials for fostamatinib as an ITP treatment in August and October 2016. The study is the second Phase 3, multi-center, randomized, double-blind, placebo controlled, study of fostamatinib disodium in the treatment of persistent/chronic immune thrombocytopenic purpura that Rigel has conducted. Primary outcome measures are defined as a stable platelet response by the end of the study (week 24) of at least 50,000/µL on at least 4 of the 6 visits between weeks 14-24. Participants received either a placebo, 100 mg, or 150 mg of the drug in the morning and evening for 24 full weeks. The first study, FIT 1 (047) met the primary endpoint in a statistically significant manner, with 18% of patients hitting the 50,000 platelets/µL of blood and no patients receiving the placebo meeting that criteria. As of June 2016, the open-label, long term extension study (049) is currently tracking 118 patients who opted to receive fostamatinib after completing either study 047 or 048.[8]
Autoimmune Hemolytic Anemia (AIHA)
Approval for treatment of autoimmune hemolytic anemia (AIHA) is in Stage 1 of Phase II trials. This study is a Phase 2, multi-center, open label, Simon two-stage study to evaluate the safety and efficacy of fostamatinib disodium in the treatment of warm antibody autoimmune hemolytic anemia. Primary outcome measures examined include a hemoglobin response measured by levels higher than 10 g/dL and 2 g/dL higher than the baseline hemoglobin. Responses were studied for a period of 12 weeks and for a dose of 150 mg in the morning and evening. The study began in April 2016 and is estimated to conclude in September 2017. The study is currently recruiting participants from U.S. states including Arizona, California, D.C., Massachusetts, New York, North Carolina, and Texas. Subjects must have had a diagnosis of primary or secondary warm antibody AIHA, and must have failed at least 1 prior treatment regimen for AIHA. Subjects cannot have a platelet count less than 30,000/µL, have AIHA secondary to autoimmune disease, have uncontrolled or poorly controlled hypertension, or have cold antibody AIHA, cold agglutinin syndrome, mixed type AIHA, or paroxysmal cold hemoglobinuria.[9]
Immunoglobulin A Nephropathy (IgAN)
Fostamatinib as a treatment for IgA nephropathy (IgAN) is in Phase II trials, which will conclude at the end of 2016. IgAN is a chronic autoimmune disease associated with inflammation in the kidneys that reduces their ability to successfully filter blood. There are currently no disease-targeted therapies for IgAN. Participants are currently being recruited from the US, Austria, Germany, Hong Kong, Taiwan, and the UK. Patients must be between 18 and 70 years old, have renal biopsy findings consistent with IgA nephropathy, have been treated with an Angiotensin Converting Enzyme inhibitor (ACEi) and/or an Angiotensin II Receptor Blocker (ARB) for at least 90 days at the maximum approved dose, have a proteinuria > 1 gm/day at diagnosis of IgA nephropathy and a level > 0.5 gm/day at the second screening visit, and a blood pressure controlled to ≤ 1302/80 with angiotensin blockade. Eligible candidates cannot have recently used cyclophosphamide, mycophenolate mofetil, azathioprine, Rituximab, or > 15 mg/day of prednisone or any other corticosteroid equivalent. The study investigates whether fostamatinib is a safe and effective treatment for IgAN. It is a Phase 2, multi-center, randomized, double-blind, ascending-dose, placebo-controlled clinical study. Primary outcome measures include the mean change in proteinuria as measured by spot urine protein/creatinine ratio (sPCR). Effects were evaluated for 100 mg, 150 mg, and placebo formulations taken twice daily by mouth for 24 weeks. The study began in October 2014 and is expected to complete by June 2017.[10]
Rigel Pharmaceuticals, Inc.
Rigel Pharmaceuticals, Inc. is a clinical stage biotech company researching the development and discovery of targeted drugs in the areas of immunology, oncology, and immune-oncology. Their business strategy is to transition a commercial-stage company with Phase 3 results from fostamatinib in ITP. The company completed and reported results from two Phase 3 clinical trials of fostamatinib in ITP in August and October 2016. These studies provide the groundwork for an NDA the company intends to submit to the US FDA in the first quarter of 2017.[11]
Rigel shares are traded on the Nasdaq Global Market under the ticker symbol RIGL. The company was first incorporated in Delaware in June 1996. Rigel went public in November of 2000. Financings were completed in January 2002, June 2003, February 2004, July 2005, May 2007, February 2008, September 2009, and June 2011. In October 2012, Rigel completed a public offering of 15,237,750 shares at $9.50 a share. Jefferies & Company, Inc. and J.P. Morgan Securities LLC acted as joint book-running managers in the offering. Rigel does not offer a direct stock purchase or dividend reinvestment program. Shares may be purchased through a broker of choice. They currently intend to retain any future earnings to fund the development and expansion of its business, therefore they do not anticipate paying cash dividends on its common stock in the near future. Rigel had approximately 98.9 million shares outstanding as of September 30, 2016.[12]
Commercialization
On June 4, 2013, Astra Zeneca announced they were giving up future development on the compound, and terminated their license with Rigel after early results from a Phase IIb study for rheumatoid arthritis. Astra Zeneca is estimated to lose $140 million as part of the contract termination.[13] On September 15, 2016, Rigel Pharmaceuticals announced plans to restructure their organization around supporting their launch of fostamatinib for the treatment of ITP. Rigel eliminated 46 positions in their research division, reducing their total workforce by 38%. The reduction is estimated to save $20 million annually. Restructured charges totaled around $5.8 million, with $5 million representing costs associated with severance pay.
One of Rigel's co-founders, Donald G. Payan, M.D. also retired from the board of directors and relinquished his position as Executive VP of Discovery and Research. Eldon C. Mayer will join as Executive VP and Chief Commercial Officer (CCO) to head the fostamatinib launch. Mr. Mayer previously held leadership with Questcor Pharmaceuticals, Connetics Corporation, Chiron Corporation, Alza Corporation, and Schering-Plough. Esteban Masuda, Ph.D was appointed Senior VP of Research to direct the downsized research group effort. Joe Lasaga as the VP of Business Development and Alliance Management and will lead Rigel's efforts to procure ex-U.S. partnerships for fostamatinib.
For the third quarter of 2016, Rigel reported a net loss of $0.24 per share, which translates to $22.6 million total. This is up from last year’s net loss of $6.7 million in the same period. Rigel reported a net YTD loss of $53.6 million for the 9 months ending on September 30, 2016, up from $38.8 million in 2015. In September 2016, Rigel announced it had reduced its workforce by 46 heads. Rigel recorded charges associated with restructuring in the third quarter of 2016 that totaled $5.8 million. $5 million of those costs covered severances, $319,000 for the impairment of certain property and equipment, and $499,000 of non-cash stock-based compensation expense which resulted from the modification of Rigel’s previous executive’s stock options.
Rigel reported cash, cash equivalents, and short-term investments of $85.3 million, compared to $126.3 million in 2015. The company expects that this amount will be able to fund their operations through the end of 2017.[14]
External links
- Rigel Pharmaceuticals http://www.rigel.com/rigel/ITP
References
- 1 2 S.P. McAdoo; F.W.K. Tam (2011). "Fostamatinib Disodium". Drugs of the Future. 36 (4): 273–280.
- ↑ Braselmann, S; Taylor, V; Zhao, H; Wang, S; Sylvain, C; Baluom, M; Qu, K; Herlaar, E; et al. (2006). "R406, an orally available spleen tyrosine kinase inhibitor blocks fc receptor signaling and reduces immune complex-mediated inflammation". The Journal of Pharmacology and Experimental Therapeutics. 319 (3): 998–1008. PMID 16946104. doi:10.1124/jpet.106.109058.
- ↑ Linger, Rachel M. A.; Keating, Amy K.; Earp, H. Shelton; Graham, Douglas K. (2008-01-01). "TAM Receptor Tyrosine Kinases: Biologic Functions, Signaling, and Potential Therapeutic Targeting in Human Cancer". Advances in cancer research. 100: 35–83. ISSN 0065-230X. PMC 3133732
 . PMID 18620092. doi:10.1016/S0065-230X(08)00002-X.
. PMID 18620092. doi:10.1016/S0065-230X(08)00002-X. - ↑ Morales-Torres, Jorge (2010). "R788 (fostamatinib disodium): a novel approach for the treatment of rheumatoid arthritis". International Journal of Clinical Rheumatology. 5 (1): 9–15. doi:10.2217/ijr.09.69.
- ↑ "Pilot Study of Fostamatinib Disodium/R935788 for the Treatment of Adult Refractory Immune Thrombocytopenic Purpura (ITP) - Full Text View - ClinicalTrials.gov". clinicaltrials.gov. Retrieved 2016-11-19.
- ↑ "Fostamatinib Shown to Be Safe but Not Effective in Rheumatoid Arthritis Patients Unresponsive to Biologic Agents". Science Daily. Jan 27, 2011. Retrieved Dec 13, 2012.
- ↑ Podolanczuk, Anna; Lazarus, Alan H.; Crow, Andrew R.; Grossbard, Elliot; Bussel, James B. (2009-04-02). "Of mice and men: an open-label pilot study for treatment of immune thrombocytopenic purpura by an inhibitor of Syk". Blood. 113 (14): 3154–3160. ISSN 0006-4971. PMID 19096013. doi:10.1182/blood-2008-07-166439.
- ↑ "A Efficacy and Safety Study of Fostamatinib in the Treatment of Persistent/Chronic Immune Thrombocytopenic Purpura (ITP) - Full Text View - ClinicalTrials.gov". clinicaltrials.gov. Retrieved 2016-11-19.
- ↑ "A Safety and Efficacy Study of R935788 in the Treatment of Warm Antibody Autoimmune Hemolytic Anemia (AIHA) - Full Text View - ClinicalTrials.gov". clinicaltrials.gov. Retrieved 2016-11-19.
- ↑ "Safety and Efficacy Study of Fostamatinib to Treat Immunoglobin A (IgA) Nephropathy - Full Text View - ClinicalTrials.gov". clinicaltrials.gov. Retrieved 2016-11-19.
- ↑ "2016 News Releases | Investors | Rigel". ir.rigel.com. Retrieved 2016-11-19.
- ↑ "SEC Filings | Investors | Rigel". ir.rigel.com. Retrieved 2016-11-19.
- ↑ Rigel to cut 30 jobs, focus on three drug programs, Sept 5th 2013, http://www.bizjournals.com/sanfrancisco/blog/biotech/2013/09/rigel-fostamatinib-itp-rigl-layoffs.html
- ↑ "Rigel Announces Third Quarter 2016 Financial Results and Provides Company Update - NASDAQ.com". NASDAQ.com. Retrieved 2016-11-19.