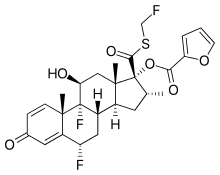
Fluticasone furoate
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Pregnancy category |
|
| Routes of administration | Intranasal |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 0.51% (Intranasal) |
| Protein binding | 91% |
| Metabolism |
Intranasal Hepatic (CYP3A4-mediated) |
| Biological half-life | 10 hours |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.158.130 |
| Chemical and physical data | |
| Formula | C27H29F3O6S |
| Molar mass | 538.576 g/mol |
| 3D model (JSmol) | |
| |
| |
Fluticasone furoate is a synthetic corticosteroid derived from fluticasone, marketed by GlaxoSmithKline as Veramyst and Flonase Sensimist (US), Allermist (Japan, アラミスト) and Avamys (Australia, Canada, EU, South Africa, South America, Mexico, Israel, and South Korea) for the treatment of allergic rhinitis administered by a nasal spray.[1]
The combination drug fluticasone furoate/vilanterol, marketed as Breo Ellipta (US, Canada) and Relvar Ellipta (UK), is approved for use in the United States by the Food and Drug Administration for long-term maintenance treatment of airflow obstruction in patients with COPD, including chronic bronchitis and emphysema.[2] As of 2015, it is also approved for the treatment of asthma. [3]
See also
References
- ↑ Bruni FM, De Luca G, Venturoli V, Boner AL (2009). "Intranasal corticosteroids and adrenal suppression". Neuroimmunomodulation. 16 (5): 353–62. PMID 19571596. doi:10.1159/000216193.
- ↑ "FDA approves Breo Ellipta to treat chronic obstructive pulmonary disease". Food and Drug Administration. May 10, 2013.
- ↑ "BREO® ELLIPTA® 100/25 (fluticasone furoate 100 mcg and vilanterol 25 mcg inhalation powder)" (PDF).
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.