Fluorenylmethyloxycarbonyl chloride
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Chloroformic acid 9H-fluoren-9-ylmethyl ester | |
| Other names
9-Fluorenylmethyl chloroformate; 9-Fluorenylmethoxycarbonyl chloride; Fmoc-chloride | |
| Identifiers | |
| 3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.044.816 |
| PubChem CID |
|
| |
| |
| Properties | |
| C15H11ClO2 | |
| Molar mass | 258.70 g·mol−1 |
| Melting point | 62 to 64 °C (144 to 147 °F; 335 to 337 K) |
| Hazards | |
| EU classification (DSD) (outdated) |
Corrosive (C) |
| R-phrases (outdated) | R34 |
| S-phrases (outdated) | S26 S36/37/39 S45 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Fluorenylmethyloxycarbonyl chloride (FMOC-Cl) is a chloroformate ester. It is used to introduce FMOC group as the FMOC carbamate.
Preparation
This compound may be prepared by reacting 9-fluorenylmethanol with phosgene:[2]
Reactions
The FMOC carbamate is often used as a protecting group for amines. The FMOC group can be introduced by reacting the amine with FMOC-Cl, e.g.:[3]
The other common method for introducing the FMOC group is through 9-fluorenylmethylsuccinimidyl carbonate (FMOC-OSu), which may itself be obtained by the reaction of FMOC-Cl with the dicyclohexylammonium salt of N-hydroxysuccinimide.[4]
It may be cleaved by bases, typically a solution of piperidine:
FMOC protection has found significant use in solid phase peptide synthesis because its removal with piperidine solution does not disturb the acid labile linker between the peptide and the resin.[5]
Because the fluorenyl group is highly fluorescent, certain UV-inactive compounds may be reacted to give the FMOC derivatives, suitable for analysis by reversed phase HPLC. Analytical uses of FMOC-Cl that do not use chromatography may be limited by the requirement that excess FMOC-Cl be removed before an analysis of fluorescence.
Amine Protection - Fluorenylmethyloxycarbonyl (Fmoc)
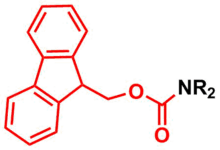
Fluorenylmethyloxycarbonyl (Fmoc) group is used as a protecting group for amines in organic synthesis.
Common amine protection methods
- Fluorenylmethyloxycarbonyl chloride or 9-fluorenylmethyloxycarbonyl azide (itself made by reacting FMOC-Cl with sodium azide), sodium bicarbonate and aqueous dioxane[6]
Common amine deprotection methods
- 20% Piperidine in dimethylformamide (Fmoc Group has an approximate half life of 6 seconds in this solution)[7]
- Tetra-n-butylammonium fluoride in the presence of dimethylformamide[8]
References
- ↑ Fmoc chloride at Sigma-Aldrich
- ↑ Carpino, Louis A.; Han, Grace Y. (1972). "9-Fluorenylmethoxycarbonyl amino-protecting group". The Journal of Organic Chemistry. 37 (22): 3404. doi:10.1021/jo00795a005.
- ↑ Yamada, Kazuhiko; Hashizume, Daisuke; Shimizu, Tadashi; Ohki, Shinobu; Yokoyama, Shigeyuki (2008). "A solid-state 17O NMR, X-ray, and quantum chemical study of N-α-Fmoc-protected amino acids". Journal of Molecular Structure. 888: 187. doi:10.1016/j.molstruc.2007.11.059.
- ↑ Paquet, A. (1982). "Introduction of 9-fluorenylmethyloxycarbonyl, trichloroethoxycarbonyl, and benzyloxycarbonyl amine protecting groups into O-unprotected hydroxyamino acids using succinimidyl carbonates". Canadian Journal of Chemistry. 60 (8): 976. doi:10.1139/v82-146.
- ↑ J. Jones, Amino Acid and Peptide Synthesis, 2nd edn., Oxford University Press, 2002.
- ↑ Carpino, Louis A.; Han, Grace Y. "9-Fluorenylmethoxycarbonyl amino-protecting group". The Journal of Organic Chemistry. 37 (22): 3404–3409. doi:10.1021/jo00795a005.
- ↑ Wuts, P; Green, T (2006); "Greene's Protective Groups in Organic Synthesis"; DOI: 10.1002/9780470053485.
- ↑ Farrera-Sinfreu, Josep; Royo, Miriam; Albericio, Fernando (2002-10-21). "Undesired removal of the Fmoc group by the free ε-amino function of a lysine residue". Tetrahedron Letters. 43 (43): 7813–7815. doi:10.1016/S0040-4039(02)01605-2.


