Febrifugine
 | |
| Names | |
|---|---|
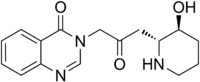
| IUPAC name
3-{3-[(2R,3S)-3-Hydroxypiperidin-2-yl]-2-oxopropyl}quinazolin-4(3H)-one | |
| Identifiers | |
| 3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.208.679 |
| PubChem CID |
|
| |
| |
| Properties | |
| C16H19N3O3 | |
| Molar mass | 301.35 g·mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Febrifugine is a quinazolinone alkaloid first isolated from the Chinese herb Dichroa febrifuga, but also found in the garden plant Hydrangea.[1] Laboratory synthesis of febrifugine determined that the originally reported stereochemistry was incorrect.[2]
Febrifugine has antimalarial properties and the synthetic halogenated derivative halofuginone is used in veterinary medicine as a coccidiostat. Other synthetic febrifugine derivatives have been used against malaria, cancer, fibrosis, and inflammatory disease.[3]
References
- ↑ McLaughlin, N. P.; Evans, P. (2010). "Dihydroxylation of Vinyl Sulfones: Stereoselective Synthesis of (+)- and (−)-Febrifugine and Halofuginone". The Journal of Organic Chemistry. 75 (2): 518–521. PMID 20000346. doi:10.1021/jo902396m.
- ↑ Kobayashi, Shū; Ueno, Masaharu; Suzuki, Ritsu; Ishitani, Haruro; Kim, Hye-Sook; Wataya, Yusuke (1999). "Catalytic Asymmetric Synthesis of Antimalarial Alkaloids Febrifugine and Isofebrifugine and Their Biological Activity". The Journal of Organic Chemistry. 64 (18): 6833. PMID 11674693. doi:10.1021/jo990877k.
- ↑ Keller, Tracy L; Zocco, Davide; Sundrud, Mark S; Hendrick, Margaret; Edenius, Maja; Yum, Jinah; Kim, Yeon-Jin; Lee, Hak-Kyo; et al. (2012). "Halofuginone and other febrifugine derivatives inhibit prolyl-tRNA synthetase". Nature Chemical Biology. 8 (3): 311–317. PMC 3281520
 . PMID 22327401. doi:10.1038/nchembio.790.
. PMID 22327401. doi:10.1038/nchembio.790.
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.