Toremifene
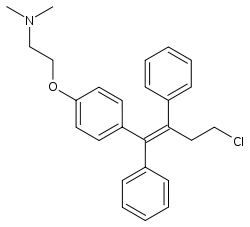 | |
| Clinical data | |
|---|---|
| Trade names | Fareston |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a608003 |
| ATC code | |
| Pharmacokinetic data | |
| Protein binding | More than 99.5% |
| Biological half-life | 5 days |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| ECHA InfoCard | 100.125.139 |
| Chemical and physical data | |
| Formula | C26H28ClNO |
| Molar mass | 405.959 g/mol |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Toremifene citrate (/ˈtoʊrɪmɪfin/) is an oral selective estrogen receptor modulator (SERM) which helps oppose the actions of estrogen in the body. Licensed in the United States under the brand name Fareston, toremifene citrate is FDA-approved for use in advanced (metastatic) breast cancer. It is also being evaluated for prevention of prostate cancer under the brand name Acapodene.[1]
In 2007 the pharmaceutical company GTx, Inc was conducting two different phase 3 clinical trials; First, a pivotal Phase clinical trial for the treatment of serious side effects of androgen deprivation therapy (ADT) (especially vertebral/spine fractures and hot flashes, lipid profile, and gynecomastia) for advanced prostate cancer, and second, a pivotal Phase III clinical trial for the prevention of prostate cancer in high risk men with high grade prostatic intraepithelial neoplasia, or PIN. Results of these trials are expected by first quarter of 2008[2]
An NDA for the first application (relief of prostate cancer ADT side effects) was submitted in Feb 2009,[3] and in Oct 2009 the FDA said they would need more clinical data, e.g. another phase III trial.[4]
References
- ↑ Price N, Sartor O, Hutson T, Mariani S. Role of 5a-reductase inhibitors and selective estrogen receptor modulators as potential chemopreventive agents for prostate cancer. Clin Prostate Cancer 2005;3:211-4. PMID 15882476
- ↑ "GTx's Phase III Clinical Development of ACAPODENE on Course Following Planned Safety Review" (Press release). GTx Inc. 2007-07-12. Retrieved 2006-07-14.
- ↑ "GTx Announces Toremifene 80 mg NDA Accepted for Review by FDA" (Press release).
- ↑ "GTx and Ipsen End Prostate Cancer Collaboration due to Costs of FDA-Requested Phase III Study". 2 Mar 2011.