Fabaceae
| Fabaceae Temporal range: Palaeocene–Recent[1] | |
|---|---|
 | |
| Kudzu (Pueraria lobata) | |
| Scientific classification | |
| Kingdom: | Plantae |
| Clade: | Angiosperms |
| Clade: | Eudicots |
| Clade: | Rosids |
| Order: | Fabales |
| Family: | Fabaceae Lindl.[2] (Leguminosae Jussieu, nom. cons.).[3] |
| Type genus | |
| Faba (now included in Vicia) Mill. | |
| Subfamilies[4] | |
| |
| Diversity | |
| 730 genera and 19,400 species | |
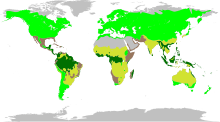 | |
| Fabaceae distribution map. Legumes are found in four major biomes: rain forest, temperate, grass, and succulent.[5] | |
| Synonyms | |
The Fabaceae, Leguminosae or Papilionaceae,[6] commonly known as the legume, pea, or bean family, are a large and economically important family of flowering plants. It includes trees, shrubs, and perennial or annual herbaceous plants, which are easily recognized by their fruit (legume) and their compound, stipulated leaves. Many legumes have characteristics of flowers and fruits. The family is widely distributed, and is the third-largest land plant family in terms of number of species, behind only the Orchidaceae and Asteraceae, with about 751 genera and some 19,000 known species.[7][8][9] The five largest of the genera are Astragalus (over 3,000 species), Acacia (over 1000 species), Indigofera (around 700 species), Crotalaria (around 700 species) and Mimosa (around 500 species), which constitute about a quarter of all legume species. The ca. 19,000 known legume species amount to about 7% of flowering plant species.[8][10] Fabaceae is the most common family found in tropical rainforests and in dry forests in the Americas and Africa.[11]
Recent molecular and morphological evidence supports the fact that the Fabaceae is a single monophyletic family.[12] This point of view has been supported not only by the degree of interrelation shown by different groups within the family compared with that found among the Leguminosae and their closest relations, but also by all the recent phylogenetic studies based on DNA sequences.[13][14][15] These studies confirm that the Fabaceae are a monophyletic group that is closely related to the Polygalaceae, Surianaceae and Quillajaceae families and that they belong to the order Fabales.[16]
Along with the cereals, some fruits and tropical roots a number of Leguminosae have been a staple human food for millennia and their use is closely related to human evolution.[17]
A number of important agricultural and food plants, including Glycine max (soybean), Phaseolus (beans), Pisum sativum (pea), Cicer arietinum (chickpeas), Medicago sativa (alfalfa), Arachis hypogaea (peanut), Ceratonia siliqua (carob), and Glycyrrhiza glabra (liquorice). A number of species are also weedy pests in different parts of the world, including: Cytisus scoparius (broom), Robinia pseudoacacia (black locust), Ulex europaeus (gorse), Pueraria lobata (kudzu), and a number of Lupinus species.
Etymology
The name 'Fabaceae' comes from the defunct genus Faba, now included in Vicia. The term "faba" comes from Latin, and appears to simply mean "bean". Leguminosae is an older name still considered valid,[6] and refers to the fruit of these plants, which are called legumes.
Description

Fabaceae range in habit from giant trees (like Koompassia excelsa) to small annual herbs, with the majority being herbaceous perennials. Plants have indeterminate inflorescences, which are sometimes reduced to a single flower. The flowers have a short hypanthium and a single carpel with a short gynophore, and after fertilization produce fruits that are legumes.
Growth habit
The Leguminosae have a wide variety of growth forms including trees, shrubs or herbaceous plants or even vines or lianas. The herbaceous plants can be annuals, biennials or perennials, without basal or terminal leaf aggregations. Many Legumes have tendrils.They are upright plants, epiphytes or vines. The latter support themselves by means of shoots that twist around a support or through cauline or foliar tendrils. Plants can be heliophytes, mesophytes or xerophytes.[3][8]
Leaves
The leaves are usually alternate and compound. Most often they are even- or odd-pinnately compound (e.g. Caragana and Robinia respectively), often trifoliate (e.g. Trifolium, Medicago) and rarely palmately compound (e.g. Lupinus), in the Mimosoideae and the Caesalpinioideae commonly bipinnate (e.g. Acacia, Mimosa). They always have stipules, which can be leaf-like (e.g. Pisum), thorn-like (e.g. Robinia) or be rather inconspicuous. Leaf margins are entire or, occasionally, serrate. Both the leaves and the leaflets often have wrinkled pulvini to permit nastic movements. In some species, leaflets have evolved into tendrils (e.g. Vicia).[3][8][17]
Many species have leaves with structures that attract ants that protect the plant from herbivore insects (a form of mutualism). Extrafloral nectaries are common among the Mimosoideae and the Caesalpinioideae, and are also found in some Faboideae (e.g. Vicia sativa). In some Acacia, the modified hollow stipules are inhabited by ants and are known as domatia.
Roots
Many Fabaceae host bacteria in their roots within structures called root nodules. These bacteria, known as rhizobia, have the ability to take nitrogen gas (N2) out of the air and convert it to a form of nitrogen that is usable to the host plant ( NO3− or NH3 ). This process is called nitrogen fixation. The legume, acting as a host, and rhizobia, acting as a provider of usable nitrate, form a symbiotic relationship.
Flowers

The flowers often have five generally fused sepals and five free petals. They are generally hermaphrodite, and have a short hypanthium, usually cup shaped. There are normally ten stamens and one elongated superior ovary, with a curved style. They are usually arranged in indeterminate inflorescences. Fabaceae are typically entomophilous plants (i.e. they are pollinated by insects), and the flowers are usually showy to attract pollinators.
In the Caesalpinioideae, the flowers are often zygomorphic, as in Cercis, or nearly symmetrical with five equal petals in Bauhinia. The upper petal is the innermost one, unlike in the Faboideae. Some species, like some in the genus Senna, have asymmetric flowers, with one of the lower petals larger than the opposing one, and the style bent to one side. The calyx, corolla, or stamens can be showy in this group.
In the Mimosoideae, the flowers are actinomorphic and arranged in globose inflorescences. The petals are small and the stamens, which can be more than just 10, have long, coloured filaments, which are the showiest part of the flower. All of the flowers in an inflorescence open at once.
In the Faboideae, the flowers are zygomorphic, and have a specialized structure. The upper petal, called the banner, is large and envelops the rest of the petals in bud, often reflexing when the flower blooms. The two adjacent petals, the wings, surround the two bottom petals. The two bottom petals are fused together at the apex (remaining free at the base), forming a boat-like structure called the keel. The stamens are always ten in number, and their filaments can be fused in various configurations, often in a group of nine stamens plus one separate stamen. Various genes in the CYCLOIDEA (CYC)/DICHOTOMA (DICH) family are expressed in the upper (also called dorsal or adaxial) petal; in some species, such as Cadia, these genes are expressed throughout the flower, producing a radially symmetrical flower.[18]
Fruit

The ovary most typically develops into a legume. A legume is a simple dry fruit that usually dehisces (opens along a seam) on two sides. A common name for this type of fruit is a "pod", although that can also be applied to a few other fruit types. A few species have evolved samarae, loments, follicles, indehiscent legumes, achenes, drupes, and berries from the basic legume fruit.
Physiology and biochemistry
The Leguminosae are rarely cyanogenic, however, where they are, the cyanogenic compounds are derived from tyrosine, phenylalanine or leucine. They frequently contain alkaloids. Proanthocyanidins can be present either as cyanidin or delphinidine or both at the same time. Flavonoids such as kaempferol, quercitin and myricetin are often present. Ellagic acid has never been found in any of the genera or species analysed. Sugars are transported within the plants in the form of sucrose. C3 photosynthesis has been found in a wide variety of genera.[3] The family has also evolved a unique chemistry. Many legumes contain toxic and indigestible substances which may be removed through various processing methods. Pterocarpans are a class of molecules (derivatives of isoflavonoids) found only in the Fabaceae.
Ecology
Distribution and habitat
The Fabaceae have an essentially worldwide distribution, being found everywhere except Antarctica and the high arctic.[9] The trees are often found in tropical regions, while the herbaceous plants and shrubs are predominant outside the tropics.[3]
Biological nitrogen fixation

.jpg)
Biological nitrogen fixation (BNF, performed by the organisms called diazotrophs) is a very old process that probably originated in the Archean eon when the primitive atmosphere lacked oxygen. It is only carried out by Euryarchaeota and just 6 of the more than 50 phyla of bacteria. Some of these lineages co-evolved together with the flowering plants establishing the molecular basis of a mutually beneficial symbiotic relationship. BNF is carried out in nodules that are mainly located in the root cortex, although they are occasionally located in the stem as in Sesbania rostrata. The spermatophytes that co-evolved with actinorhizal diazotrophs (Frankia) or with rhizobia to establish their symbiotic relationship belong to 11 families contained within the Rosidae clade (as established by the gene molecular phylogeny of rbcL, a gene coding for part of the RuBisCO enzyme in the chloroplast). This grouping indicates that the predisposition for forming nodules probably only arose once in flowering plants and that it can be considered as an ancestral characteristic that has been conserved or lost in certain lineages. However, such a wide distribution of families and genera within this lineage indicates that nodulation had multiple origins. Of the 10 families within the Rosidae, 8 have nodules formed by actinomyces (Betulaceae, Casuarinaceae, Coriariaceae, Datiscaceae, Elaeagnaceae, Myricaceae, Rhamnaceae and Rosaceae), and the two remaining families, Ulmaceae and Fabaceae have nodules formed by rhizobia.[19][20]
The rhizobia and their hosts must be able to recognize each other for nodule formation to commence. Rhizobia are specific to particular host species although a rhizobia species may often infect more than one host species. This means that one plant species may be infected by more than one species of bacteria. For example, nodules in Acacia senegal can contain seven species of rhizobia belonging to three different genera. The most distinctive characteristics that allow rhizobia to be distinguished apart are the rapidity of their growth and the type of root nodule that they form with their host.[20] Root nodules can be classified as being either indeterminate, cylindrical and often branched, and determinate, spherical with prominent lenticels. Indeterminate nodules are characteristic of legumes from temperate climates, while determinate nodules are commonly found in species from tropical or subtropical climates.[20]
Nodule formation is common throughout the leguminosae, it is found in the majority of its members that only form an association with rhizobia, which in turn form an exclusive symbiosis with the leguminosae (with the exception of Parasponia, the only genus of the 18 Ulmaceae genera that is capable of forming nodules). Nodule formation is present in all the leguminosae sub-families, although it is less common in the Caesalpinioideae. All types of nodule formation are present in the sub-family Papilionoideae: indeterminate (with the meristem retained), determinate (without meristem) and the type included in Aeschynomene. The latter two are thought to be the most modern and specialised type of nodule as they are only present in some lines of the Papilionoideae sub-family. Even though nodule formation is common in the two monophyletic subfamilies Papilionoideae and Mimosoideae they also contain species that do not form nodules. The presence or absence of nodule-forming species within the three sub-families indicates that nodule formation has arisen several times during the evolution of the leguminosae and that this ability has been lost in some lineages. For example, within the genus Acacia, a member of the Mimosoideae, A. pentagona does not form nodules, while other species of the same genus readily form nodules, as is the case for Acacia senegal, which forms both rapidly and slow growing rhizobial nodules.
Chemical ecology
A large number of species within many genera of leguminous plants, e.g. Astragalus, Coronilla, Hippocrepis, Indigofera, Lotus, Securigera and Scorpiurus, produce chemicals that derive from the compound 3-nitropropanoic acid (3-NPA, beta-nitropropionic acid). The free acid 3-NPA is an irreversible inhibitor of mitochondrial respiration, and thus the compound inhibits the tricarboxylic acid cycle. This inhibition caused by 3-NPA is especially toxic to nerve cells and represents a very general toxic mechanism suggesting a profound ecological importance due to the big number of species producing this compound and its derivatives. A second and closely related class of secondary metabolites that occur in many species of leguminous plants is defined by isoxazolin-5-one derivatives. These compounds occur in particular together with 3-NPA and related derivatives at the same time in the same species, as found in Astragalus canadensis and Astragalus collinus. 3-NPA and isoxazlin-5-one derivatives also occur in many species of leaf beetles (see defense in insects).[21]
Evolution, phylogeny and taxonomy
Evolution
The order Fabales contains around 7.3% of eudicot species and the greatest part of this diversity is contained in just one of the four families that order contains: Fabaceae. This clade also includes the Polygalaceae, Surianaceae and Quillajaceae families and its origins date back 94 to 89 million years, although it started its diversification some 79 to 74 million years ago.[9] In fact, the Fabaceae have diversified during the early tertiary to become a ubiquitous part of the modern earth’s biota, along with many other families belonging to the flowering plants.[12][22]
The Fabaceae have an abundant and diverse fossil record, especially for the Tertiary period. Fossils of flowers, fruit, leaves, wood and pollen from this period have been found in numerous locations.[23][24][25][26][27] The earliest fossils that can be definitively assigned to the Fabaceae appeared in the late Palaeocene (approximately 56 million years ago).[28][29] Representatives of the 3 sub-families traditionally recognised as being members of the Fabaceae – Cesalpinioideae, Papilionoideae and Mimosoideae — as well as members of the large clades within these sub-families – such as the genistoides – have been found in periods a little later, starting between 55 and 50 million years ago.[22] In fact, a wide variety of taxa representing the main lineages in the Fabaceae have been found in the fossil record dating from the middle to the late Eocene, suggesting that the majority of the modern Fabaceae groups were already present and that a broad diversification occurred during this period.[22] Therefore, the Fabaceae started their diversification approximately 60 million years ago and the most important clades separated some 50 million years ago.[30] The age of the main Cesalpinioideae clades have been estimated as between 56 and 34 million years and the basal group of the Mimosoideae as 44 ± 2.6 million years.[31][32] The division between Mimosoideae and Faboideae is dated as occurring between 59 and 34 million years ago and the basal group of the Faboideae as 58.6 ± 0.2 million years ago.[33] It has been possible to date the divergence of some of the groups within the Faboideae, even though diversification within each genus was relatively recent. For instance, Astragalus separated from the Oxytropis some 16 to 12 million years ago. In addition, the separation of the aneuploid species of Neoastragalus started 4 million years ago. Inga, another genus of the Papilionoideae with approximately 350 species, seems to have diverged in the last 2 million years.[34][35][36][37]
It has been suggested, based on fossil and phylogenetic evidence, that legumes originally evolved in arid and/or semi-arid regions along the Tethys seaway during the Palaeogene Period.[5][38] However, others contend that Africa (or even the Americas) cannot yet be ruled out as the origin of the family.[39][40]
The current hypothesis about the evolution of the genes needed for nodulation is that they were recruited from other pathways after a polyploidy event.[41] Several different pathways have been implicated as donating duplicated genes to the pathways need for nodulation. The main donors to the pathway were the genes associated with the arbuscular mycorrhiza symbiosis genes, the pollen tube formation genes and the haemoglobin genes. One of the main genes shown to be shared between the arbuscular mycorrhiza pathway and the nodulation pathway is SYMRK and it is involved in the plant-bacterial recognition.[42] The pollen tube growth is similar to the infection thread development in that infection threads grow in a polar manner that is similar to a pollen tubes polar growth towards the ovules. Both pathways include the same type of enzymes, pectin-degrading cell wall enzymes.[43] The enzymes needed to reduce nitrogen, nitrogenases, require a substantial input of ATP but at the same time are sensitive to free oxygen. To meet the requirements of this paradoxical situation, the plants express a type of haemoglobin called leghaemoglobin that is believed to be recruited after a duplication event.[44] These three genetic pathways are believed to be part of a gene duplication event then recruited to work in nodulation.
Phylogeny and taxonomy
Phylogeny
The phylogeny of the legumes has been the object of many studies by research groups from around the world. These studies have used morphology, DNA data (the chloroplast intron trnL, the chloroplast genes rbcL and matK, or the ribosomal spacers ITS) and cladistic analysis in order to investigate the relationships between the family’s different lineages. Fabaceae is consistently recovered as monophyletic.[45] The studies further confirmed that the traditional subfamilies Mimosoideae and Papilionoideae were each monophyletic but both were nested within the paraphyletic subfamily Caesalpinioideae.[1][45] All the different approaches yielded similar results regarding the relationships between the family's main clades.[9][46][47][48][49][50][51][52][53] Following extensive discussion in the legume phylogenetics community, the Legume Phylogeny Working Group reclassified Fabaceae into six subfamilies, which necessitated the segregation of four new subfamilies from Caesalpinioideae and merging Caesapinioideae sensu stricto with the former subfamily Mimosoideae.[4]
| Fabales |
| |||||||||||||||||||||||||||||||||||||||||||||
Taxonomy
The Fabaceae are placed in the order Fabales according to most taxonomic systems, including the APG III system.[2] The family now includes six subfamilies:[4]
- Cercidoideae: 12 genera and ~335 species. Mainly tropical. Bauhinia, Cercis.
- Detarioideae: 84 genera and ~760 species. Mainly tropical. Amherstia, Detarium, Tamarindus.
- Duparquetioideae: 1 genus and 1 species. West and Central Africa. Duparquetia.
- Dialioideae: 17 genera and ~85 species. Widespread throughout the tropics. Dialium.
- Caesalpinioideae: 148 genera and ~4400 species. Pantropical. Caesalpinia, Senna, Mimosa, Acacia. Includes the former subfamily Mimosoideae (80 genera and ~3200 species; mostly tropical and warm temperate Asia and America).
- Faboideae (Papilionoideae[54]): 503 genera and ~14,000 species. Cosmopolitan. Astragalus, Lupinus, Pisum.
Economic and cultural importance
Legumes are economically and culturally important plants due to their extraordinary diversity and abundance, the wide variety of edible vegetables they represent and due to the variety of uses they can be put to: in horticulture and agriculture, as a food, for the compounds they contain that have medicinal uses and for the oil and fats they contain that have a variety of uses.[55][56][57][58]
Food and forage
The history of legumes is tied in closely with that of human civilization, appearing early in Asia, the Americas (the common bean, several varieties) and Europe (broad beans) by 6,000 BCE, where they became a staple, essential as a source of protein.
Their ability to fix atmospheric nitrogen reduces fertilizer costs for farmers and gardeners who grow legumes, and means that legumes can be used in a crop rotation to replenish soil that has been depleted of nitrogen. Legume seeds and foliage have a comparatively higher protein content than non-legume materials, due to the additional nitrogen that legumes receive through the process. Legumes are commonly used as natural fertilizers. Some legume species perform hydraulic lift, which makes them ideal for intercropping.[59]
Farmed legumes can belong to numerous classes, including forage, grain, blooms, pharmaceutical/industrial, fallow/green manure and timber species, with most commercially farmed species filling two or more roles simultaneously.
There are of two broad types of forage legumes. Some, like alfalfa, clover, vetch, and Arachis, are sown in pasture and grazed by livestock. Other forage legumes such as Leucaena or Albizia are woody shrub or tree species that are either broken down by livestock or regularly cut by humans to provide stock feed.
Grain legumes are cultivated for their seeds, and are also called pulses. The seeds are used for human and animal consumption or for the production of oils for industrial uses. Grain legumes include both herbaceous plants like beans, lentils, lupins, peas and peanuts.[60] and trees such as carob, mesquite and tamarind.
Bloom legume species include species such as lupin, which are farmed commercially for their blooms as well as being popular in gardens worldwide. Laburnum, Robinia, Gleditsia, Acacia, Mimosa, and Delonix are ornamental trees and shrubs.
Industrial farmed legumes include Indigofera, cultivated for the production of indigo, Acacia, for gum arabic, and Derris, for the insecticide action of rotenone, a compound it produces.
Fallow or green manure legume species are cultivated to be tilled back into the soil to exploit the high nitrogen levels found in most legumes. Numerous legumes are farmed for this purpose, including Leucaena, Cyamopsis and Sesbania.
Various legume species are farmed for timber production worldwide, including numerous Acacia species, Dalbergia species, and Castanospermum australe.
Melliferous plants offer nectar to bees and other insects to encourage them to carry pollen from the flowers of one plant to others thereby ensuring pollination.A number of legume species are good nectar providers such as alfalfa, white clover, sweet clover and various Prosopis species. Many plants in the Fabaceae family are an important source of pollen for the bumblebee species Bombus hortorum. This bee species is especially fond of one species in particular; Trifolium pratense, also known as red clover, is a popular food source in the diet of Bombus hortorum.[61]
Industrial uses
Natural gums
Natural gums are vegetable exudates that are released as the result of damage to the plant such as that resulting from the attack of an insect or a natural or artificial cut. These exudates contain heterogeneous polysaccharides formed of different sugars and usually containing uronic acids. They form viscous colloidal solutions. There are different species that produce gums. The most important of these species belong to the leguminosae. They are widely used in the pharmaceutical, cosmetic, food and textile sectors. They also have interesting therapeutic properties; for example gum arabic is antitussive and anti-inflammatory. The most well known gums are tragacanth (Astragalus gummifer), gum arabic (Acacia senegal) and guar gum (Cyamopsis tetragonoloba).[62]
Dyes

The species used to produce dyes include the following: Logwood Haematoxylon campechianum; a large spiny tree that can grow up to 15 m tall. Its cork is thin and soft and its wood is hard. The heartwood is used to produce dyes that are red and purple. The histological stain called haematoxylin is produced from this species. Brazilwood tree (Caesalpinia echinata) is similar to the previous tree but smaller and with red or purple flowers. The wood is also used to produce a red or purple dye. The Madras thorn (Pithecallobium dulce) is another spiny tree native to Latin America, it grows up to 4 m high and has yellow or green flowers that grow in florets. Its fruit is reddish and is used to produce a yellow dye.[63] Indigo dye is extracted from the True indigo plant Indigofera tinctoria that is native to Asia. In Central and South America dyes are produced from two species related to this species, indigo from Indigofera suffruticosa and Natal indigo from Indigofera arrecta.yellow dye is extracted from Butea monosperma commonly called as flame of the forest.
Ornamentals

Legumes have been used as ornamental plants throughout the world for many centuries. Their vast diversity of heights, shapes, foliage and flower colour means that this family is commonly used in the design and planting of everything from small gardens to large parks.[17] The following is a list of the main ornamental legume species, listed by sub-family.
- Subfamily Caesalpinioideae: Bauhinia forficata, Caesalpinia gilliesii, Caesalpinia spinosa, Ceratonia siliqua, Cercis siliquastrum, Gleditsia triacanthos, Gymnocladus dioica, Parkinsonia aculeata, Senna multiglandulosa.[64]
- Subfamily Mimosoideae: Acacia caven, Acacia cultriformis, Acacia dealbata, Acacia karroo, Acacia longifolia, Acacia melanoxylon, Acacia paradoxa, Acacia retinodes, Acacia saligna, Acacia verticillata, Acacia visco, Albizzia julibrissin, Calliandra tweediei, Paraserianthes lophantha, Prosopis chilensis.[64]
- Subfamily Faboideae: Clianthus puniceus, Citysus scoparius, Erythrina crista-galli, Erythrina falcata, Laburnum anagyroides, Lotus peliorhynchus, Lupinus arboreus, Lupinus polyphyllus, Otholobium glandulosum, Retama monosperma, Robinia hispida, Robinia luxurians, Robinia pseudoacacia, Sophora japonica, Sophora macnabiana, Sophora macrocarpa, Spartium junceum, Teline monspessulana, Tipuana tipu, Wisteria sinensis.[64]
Emblematic Leguminosae
- The Cockspur Coral Tree (Erythrina crista-galli), is the National Flower of Argentina and Uruguay.[65]
- The Elephant ear tree (Enterolobium cyclocarpum) is the national tree of Costa Rica, by Executive Order of 31 August 1959.[66]
- The Brazilwood tree (Caesalpinia echinata) has been the national tree of Brazil since 1978.[67]
- The Golden wattle Acacia pycnantha is Australia’s national flower.[68]
- The Hong Kong Orchid tree Bauhinia blakeana is the national flower of Hong Kong.[69]
Image gallery
 Acacia baileyana (Wattle)
Acacia baileyana (Wattle) Loments of Alysicarpus vaginalis
Loments of Alysicarpus vaginalis- Calliandra emarginata

_in_Hyderabad%2C_AP_W2_IMG_9903.jpg) Dichrostachys cinerea Sickle Bush
Dichrostachys cinerea Sickle Bush- Indigofera gerardiana
 Tendrils of Lathyrus odoratus (Sweet pea)
Tendrils of Lathyrus odoratus (Sweet pea) Inflorescence of Lupinus arboreus (Yellow bush lupin)
Inflorescence of Lupinus arboreus (Yellow bush lupin) Pisum sativum (Peas); note the leaf-like stipules
Pisum sativum (Peas); note the leaf-like stipules Smithia conferta
Smithia conferta
 Kashubian vetch – Kashubia
Kashubian vetch – Kashubia Zornia gibbosa
Zornia gibbosa
References
- 1 2 Wojciechowski, M. F.; Lavin, M.; Sanderson, M. J. (2004). "A phylogeny of legumes (Leguminosae) based on analysis of the plastid matK gene resolves many well-supported sub clades within the family". American Journal of Botany. 91 (11): 1846–62. PMID 21652332. doi:10.3732/ajb.91.11.1846.
- 1 2 Angiosperm Phylogeny Group (2009). "An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG III" (PDF). Botanical Journal of the Linnean Society. 161 (2): 105–121. doi:10.1111/j.1095-8339.2009.00996.x. Retrieved 4 February 2014.
- 1 2 3 4 5 Watson L.; Dallwitz, M. J. (2007-06-01). "The families of flowering plants: Leguminosae". Retrieved 9 February 2008.
- 1 2 3 The Legume Phylogeny Working Group (LPWG). (2017). "A new subfamily classification of the Leguminosae based on a taxonomically comprehensive phylogeny". Taxon. 66 (1): 44–77. doi:10.12705/661.3.
- 1 2 Schrire, B. D.; Lewis, G. P.; Lavin, M. (2005). "Biogeography of the Leguminosae". In Lewis, G; Schrire, G.; Mackinder, B.; Lock, M. Legumes of the world. Kew, England: Royal Botanic Gardens. pp. 21–54. ISBN 1-900347-80-6.
- 1 2 International Code of Nomenclature for algae, fungi, and plants. Article 18.5 states: "The following names, of long usage, are treated as validly published: ....Leguminosae (nom. alt.: Fabaceae; type: Faba Mill. [= Vicia L.]); ... When the Papilionaceae are regarded as a family distinct from the remainder of the Leguminosae, the name Papilionaceae is conserved against Leguminosae." English pronunciations are as follows: English: /fəˈbeɪsi, -siˌaɪ, -siˌeɪ, -siˌi/; English: /ləˌɡjuːməˈnoʊsi/; and English: /pəˌpɪlioʊˈneɪsiˌi/.
- ↑ Christenhusz, M. J. M.; Byng, J. W. (2016). "The number of known plants species in the world and its annual increase". Phytotaxa. Magnolia Press. 261 (3): 201–217. doi:10.11646/phytotaxa.261.3.1.
- 1 2 3 4 Judd, W. S., Campbell, C. S. Kellogg, E. A. Stevens, P.F. Donoghue, M. J. (2002), Plant systematics: a phylogenetic approach, Sinauer Axxoc, 287-292. ISBN 0-87893-403-0.
- 1 2 3 4 Stevens, P. F. "Fabaceae". Angiosperm Phylogeny Website. Version 7 May 2006. Retrieved 28 April 2008.
- ↑ Magallón, S. A., and Sanderson, M. J.; Sanderson (2001). "Absolute diversification rates in angiosperm clades" (PDF). Evolution. 55 (9): 1762–1780. PMID 11681732. doi:10.1111/j.0014-3820.2001.tb00826.x.
- ↑ Burnham, R. J.; Johnson, K. R. (2004). "South American palaeobotany and the origins of neotropical rainforests". Philosophical Transactions of the Royal Society B: Biological Sciences. 359 (1450): 1595–1610. PMC 1693437
 . PMID 15519975. doi:10.1098/rstb.2004.1531.
. PMID 15519975. doi:10.1098/rstb.2004.1531. - 1 2 Lewis G., Schrire B., Mackinder B. and Lock M. 2005. (eds.) Legumes of the world. The Royal Botanic Gardens, Kew, Reino Unido. 577 pages. 2005. ISBN 1-900347-80-6.
- ↑ Doyle, J. J., J. A. Chappill, C.D. Bailey, & T. Kajita. 2000. Towards a comprehensive phylogeny of legumes: evidence from rbcL sequences and non-molecular data. pp. 1 -20 in Advances in legume systematics, part 9, (P. S. Herendeen and A. Bruneau, eds.). Royal Botanic Gardens, Kew, UK.
- ↑ Kajita, T.; Ohashi, H.; Tateishi, Y.; Bailey, C. D.; Doyle, J. J. (2001). "rbcL and legume phylogeny, with particular reference to Phaseoleae, Millettieae, and allies". Systematic Botany. 26: 515–536. JSTOR 3093979.
- ↑ Wojciechowski, M. F., M. Lavin and M. J. Sanderson; Lavin; Sanderson (2004). "A phylogeny of legumes (Leguminosae) based on analysis of the plastid matK gene resolves many well-supported sub clades within the family". American Journal of Botany. 91 (11): 1846–1862. PMID 21652332. doi:10.3732/ajb.91.11.1846.
- ↑ Angiosperm Phylogeny Group [APG] (2003). "An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG II" (PDF). Botanical Journal of the Linnean Society. 141: 399–436. doi:10.1046/j.1095-8339.2003.t01-1-00158.x.
- 1 2 3 Burkart, A. Leguminosas. In: Dimitri, M. 1987. Enciclopedia Argentina de Agricultura y Jardinería. Tomo I. Descripción de plantas cultivadas. Editorial ACME S.A.C.I., Buenos Aires. pages: 467-538.
- ↑ Hélène L. Citerne; R. Toby Pennington; Quentin C. B. Cronk (8 August 2006). "An apparent reversal in floral symmetry in the legume Cadia is a homeotic transformation". PNAS. 103 (32): 12017–12020. PMC 1567690
 . PMID 16880394. doi:10.1073/pnas.0600986103.
. PMID 16880394. doi:10.1073/pnas.0600986103. - ↑ Lloret, L.; Martínez-Romero, E. (2005). "Evolución y filogenia de". Rhizobium. 47 (1–2): 43–60.
- 1 2 3 Sprent, J. I. 2001. Nodulation in legumes. Royal Botanic Gardens, Kew, UK.
- ↑ Becker T.; et al. (2017). "A tale of four kingdoms - isoxazolin-5-one- and 3-nitropropanoic acid-derived natural products". Natural Product Reports. 34 (4): 343–360. doi:10.1039/C6NP00122J.
- 1 2 3 Herendeen, P. S., W. L. Crepet, and D. L. Dilcher. 1992. The fossil history of the Leguminosae: phylogenetic and biogeographic implications. Pages 303 – 316 in Advances in Legume Systematics, part 4, the fossil record (P. S. Herendeen and D .L. Dilcher, eds). Royal Botanic Gardens, Kew, UK.
- ↑ Crepet, W. L.; Taylor, D. W. (1985). "The Diversification of the Leguminosae: First Fossil Evidence of the Mimosoideae and Papilionoideae". Science. 228 (4703): 1087–1089. ISSN 0036-8075. PMID 17737903. doi:10.1126/science.228.4703.1087.
- ↑ Crepet, W. L., and D. W. Taylor (1986). "Primitive mimosoid flowers from the Palaeocene-Eocene and their systematic and evolutionary implications.". American J. Botany. 73: 548–563. JSTOR 2444261. doi:10.2307/2444261.
- ↑ Crepet, W. L., and P. S. Herendeen. 1992. Papilionoid flowers from the early Eocene of south eastern North America. Pages 43–55 in Advances in Legume Systematics, part 4, the fossil record (P. S. Herendeen and D. L. Dilcher, eds.). Royal Botanic Gardens, Kew, UK.
- ↑ Herendeen, P. S. 1992. The fossil history of Leguminosae from the Eocene of south eastern North America. Pages 85-160 in Advances in Legume Systematics, part 4, the fossil record (Herendeen, P. S., and D. L. Dilcher, eds.). Royal Botanic Gardens, Kew, UK.
- ↑ Herendeen, P. S. 2001. The fossil record of the Leguminosae: recent advances. In Legumes Down Under: the Fourth International Legume conference, Abstracts, 34–35. Australian National University, Canberra, Australia.
- ↑ Herendeen, P. S., and S. Wing. 2001. Papilionoid legume fruits and leaves from the Palaeocene of north western Wyoming. Botany 2001 Abstracts, published by Botanical Society of America (http://www.botany2001.org/).
- ↑ Wing, S. L., F. Herrera, and C. Jaramillo. 2004. A Palaeocene flora from the Cerrajón Formation, Guajíra Peninsula, north eastern Colombia. Pages 146-147 in VII International Organization of Paleobotany Conference Abstracts (21–26 March). Museo Egidio Feruglio, Trelew, Argentina.
- ↑ Bruneau, A., Lewis, G. P., Herendeen, P. S., Schrire, B., & Mercure, M. 2008b. Biogeographic patterns in early-diverging clades of the Leguminosae. Pp. 98-99, in Botany 2008. Botany without Borders. [Botanical Society of America, Abstracts.]
- ↑ Bruneau, A.; Mercure, M.; Lewis, G. P. & Herendeen, P. S. (2008). "Phylogenetic patterns and diversification in the caesalpinioid legumes". Canadian Journal of Botany. 86 (7): 697–718. doi:10.1139/B08-058.
- ↑ Lavin, M., Herendeen, P. S., y Wojciechowski, M. F.; Herendeen; Wojciechowski (2005). "Evolutionary Rates Analysis of Leguminosae Implicates a Rapid Diversification of Lineages during the Tertiary". Systematic Biology. 54 (4): 575–594. PMID 16085576. doi:10.1080/10635150590947131.
- ↑ Wikstrom, N.; Savolainen, V.; Chase, M. W. (2001). "Evolution of the angiosperms: calibrating the family tree". Proceedings of the Royal Society B: Biological Sciences. 268 (1482): 2211–2220. PMC 1088868
 . PMID 11674868. doi:10.1098/rspb.2001.1782.
. PMID 11674868. doi:10.1098/rspb.2001.1782. - ↑ Wojciechowski, M. F. 2003. Reconstructing the phylogeny of legumes (Leguminosae): An early 21st century perspective. Pp. 5-35, in Klitgaard, B. B. & Bruneau, A. (eds), Advances in Legume Systematics, Part 10, Higher Level Systematics. Royal Botanic Gardens, Kew.
- ↑ Wojciechowski, M. F. (2005). "Astragalus (Fabaceae): A molecular phylogenetic perspective". Brittonia. 57 (4): 382–396. JSTOR 4098954. doi:10.1663/0007-196X(2005)057[0382:AFAMPP]2.0.CO;2.
- ↑ Wojciechowski, M. F.; Sanderson, M. J.; Baldwin, B. G.; Donoghue, M. J. (1993). "Monophyly of aneuploid Astragalus: Evidence from nuclear ribosomal DNA internal transcribed spacer sequences". American Journal of Botany. 80: 711–722. JSTOR 2445441. doi:10.2307/2445441.
- ↑ Wojciechowski, Martin F., Johanna Mahn, and Bruce Jones. 2006. Fabaceae. legumes. Version 14 June 2006. The Tree of Life Web Project, http://tolweb.org/
- ↑ Schrire, B. D.; Lavin, M.; Lewis, G. P. (2005). "Global distribution patterns of the Leguminosae: insights from recent phylogenies". In Friis, I; Balslev, H. Plant diversity and complexity patterns: local, regional and global dimensions. Biologiske Skrifter. 55. Viborg, Denmark: Special-Trykkeriet Viborg A/S. pp. 375–422. ISBN 87-7304-304-4.
- ↑ Pan, Aaron D.; Jacobs, Bonnie F.; Herendeen, Patrick S. (2010). "Detarieae sensu lato (Fabaceae) from the Late Oligocene (27.23 Ma) Guang River flora of north-western Ethiopia". Botanical Journal of the Linnean Society. 163: 44–54. doi:10.1111/j.1095-8339.2010.01044.x.
- ↑ Doyle, J. J.; Luckow, MA (2003). "The Rest of the Iceberg. Legume Diversity and Evolution in a Phylogenetic Context". Plant Physiology. 131 (3): 900–10. PMC 1540290
 . PMID 12644643. doi:10.1104/pp.102.018150.
. PMID 12644643. doi:10.1104/pp.102.018150. - ↑ Yokota, Keisuke; Hayashi, Makoto (2011). "Function and evolution of nodulation genes in legumes". Cellular and Molecular Life Sciences. 68 (8): 1341–51. PMID 21380559. doi:10.1007/s00018-011-0651-4.
- ↑ Markmann, Katharina; Giczey, Gábor; Parniske, Martin (2008). "Functional Adaptation of a Plant Receptor- Kinase Paved the Way for the Evolution of Intracellular Root Symbioses with Bacteria". PLoS Biology. 6 (3): e68. PMC 2270324
 . PMID 18318603. doi:10.1371/journal.pbio.0060068.
. PMID 18318603. doi:10.1371/journal.pbio.0060068. - ↑ Rodríguez-Llorente, Ignacio D.; Pérez-Hormaeche, Javier; Mounadi, Kaoutar El; Dary, Mohammed; Caviedes, Miguel A.; Cosson, Viviane; Kondorosi, Adam; Ratet, Pascal; Palomares, Antonio J. (2004). "From pollen tubes to infection threads: Recruitment of Medicago floral pectic genes for symbiosis". The Plant Journal. 39 (4): 587–98. PMID 15272876. doi:10.1111/j.1365-313X.2004.02155.x.
- ↑ Downie, J. Allan (2005). "Legume Haemoglobins: Symbiotic Nitrogen Fixation Needs Bloody Nodules". Current Biology. 15 (6): R196–8. PMID 15797009. doi:10.1016/j.cub.2005.03.007.
- 1 2 Martin F. Wojciechowski; Johanna Mahn; Bruce Jones (2006). "Fabaceae". The Tree of Life Web Project.
- ↑ Käss E, Wink M (1996). "Molecular evolution of the Leguminosae: phylogeny of the three subfamilies based on rbcL sequences". Biochemical Systematics and Evolution. 24 (5): 365–378. doi:10.1016/0305-1978(96)00032-4.
- ↑ Käss E, Wink M (1997). "Phylogenetic relationships in the Papilionoideae (Family Leguminosae) based on nucleotide sequences of cpDNA (rbcL) and ncDNA (ITS1 and 2)". Mol. Phylogenet. Evol. 8 (1): 65–88. PMID 9242596. doi:10.1006/mpev.1997.0410.
- ↑ Doyle JJ, Doyle JL, Ballenger JA, Dickson EE, Kajita T, Ohashi H (1997). "A phylogeny of the chloroplast gene rbcL in the Leguminosae: taxonomic correlations and insights into the evolution of nodulation". Am. J. Bot. 84 (4): 541–554. PMID 21708606. doi:10.2307/2446030.
- ↑ Lavin M, Doyle JJ, Palmer JD (1990). "Evolutionary significance of the loss of the chloroplast-DNA inverted repeat in the Leguminosae subfamily Papilionoideae". Evolution. 44 (2): 390–402. JSTOR 2409416. doi:10.2307/2409416.
- ↑ Sanderson MJ, Wojciechowski MF (1996). "Diversification rates in a temperate legume clade: are there "so many species" of Astragalus (Fabaceae)?". Am. J. Bot. 83 (11): 1488–1502. JSTOR 2446103. doi:10.2307/2446103.
- ↑ Chappill JA. (1995). "Cladistic analysis of the Leguminosae: the development of an explicit hypothesis". In Crisp MD, Doyle JJ. Advances in Legume Systematics, Part 7: Phylogeny. Royal Botanic Gardens, Kew, UK. pp. 1–10. ISBN 9780947643799.
- ↑ Bruneau A, Mercure M, Lewis GP, Herendeen PS (2008). "Phylogenetic patterns and diversification in the caesalpinioid legumes". Botany. 86 (7): 697–718. doi:10.1139/B08-058.
- ↑ Cardoso D, Pennington RT, de Queiroz LP, Boatwright JS, Van Wykd BE, Wojciechowskie MF, Lavin M (2013). "Reconstructing the deep-branching relationships of the papilionoid legumes". S. Afr. J. Bot. 89: 58–75. doi:10.1016/j.sajb.2013.05.001.
- ↑ NOTE: The subfamilial name Papilionoideae for Faboideae is approved by the International Code of Nomenclature for algae, fungi, and plants, Article 19.8
- ↑ Allen, O. N., & E. K. Allen. 1981. The Leguminosae, A Source Book of Characteristics, Uses, and Nodulation. The University of Wisconsin Press, Madison, USA.
- ↑ Duke, J. A. 1992. Handbook of Legumes of Economic Importance. Plenum Press, New York, USA.
- ↑ Graham, P. H.; Vance, C. P. (2003). "Legumes: importance and constraints to greater use". Plant Physiology. 131: 872–877. doi:10.1104/pp.017004.
- ↑ Wojciechowski, M.F. 2006. Agriculturally & Economically Important Legumes.. Accessed 15 November 2008.
- ↑ Sprent, Janet I. (2009). Legume Nodulation: A Global Perspective. Ames, Iowa: Wiley-Blackwell. p. 12. ISBN 1-4051-8175-3. Preview available at Google Books.
- ↑ The gene bank and breeding of grain legumes (lupine, vetch, soya and beah) / B.S. Kurlovich and S.I. Repyev (Eds.), - St. Petersburg, The N.I. Vavilov Institute of Plant Industry, 1995, 438p. - (Theoretical basis of plant breeding. V.111)
- ↑ Goulson, J.; Kaden, J.C.; Lepais, G.C. (2011). "Population Structure, Dispersal and Colonization History of the Garden Bumblebee Bombus Hortorum in the Western Isles of Scotland". Conservation Genetics. 12: 867–879. doi:10.1007/s10592-011-0190-4.
- ↑ Kuklinski, C. 2000. Farmacognosia : estudio de las drogas y sustancias medicamentosas de origen natural. Ediciones Omega, Barcelona. ISBN 84-282-1191-4
- ↑ Marquez, A. C., Lara, O.F., Esquivel, R. B. & Mata, E. R. 1999. Composición, usos y actividad biológica: Plantas medicinales de México II. UNAM. First edition. México, D.F.
- 1 2 3 Macaya J. 1999. Leguminosas arbóreas y arbustivas cultivadas en Chile. Chloris Chilensis Año 2. Nº1.
- ↑ Ministerio de Educación de la Nación. Subsecretaría de Coordinación Administrativa. Día de la Flor Nacional "El Ceibo". Efemérides Culturales Argentinas. Consulted 3 March 2010.
- ↑ Gilbert Vargas Ulate. 1997. Geografía turística de Costa Rica. EUNED, 180 p. ISBN 9977-64-900-6, 9789977649009.
- ↑ "Lei Nº 6.607, de 7 de dezembro de 1978. O Presidente da República, faço saber que o Congresso Nacional decreta e eu sanciono a seguinte Lei: Art. 1º- É declarada Árvore Nacional a leguminosa denominada Pau-Brasil (Caesalpinia echinata, Lam), cuja festa será comemorada, anualmente, quando o Ministério da Educação e Cultura promoverá campanha elucidativa sobre a relevância daquela espécie vegetal na História do Brasil."
- ↑ Boden, Anne (1985). "Golden Wattle: Floral Emblem of Australia" (http). Australian National Botanic Gardens. Retrieved 8 October 2008.
- ↑ Williams, Martin (1999). "Golden Enigmatic Beauty" (http). Bahuninia. Retrieved 8 October 2008.
External links
| Wikispecies has information related to: Fabaceae |
| Wikimedia Commons has media related to Fabaceae. |
| Wikisource has the text of the 1920 Encyclopedia Americana article Leguminosæ. |
- Leguminosae at The Plant List
- Leguminosae at The Families of Flowering Plants (DELTA)
- Fabaceae at the Encyclopedia of Life
- Fabaceae at the Angiosperm Phylogeny Website
- Fabaceae at the Tree of Life Web Project
- Fabaceae at the online Flora of China
- Fabaceae at the online Guide to the Flora of Mongolia
- Fabaceae at the online Flora of Zimbabwe
- Fabaceae at the online Flora of Western Australia
- Fabaceae at the online Flora of New Zealand
- Leguminosae at the International Legume Database & Information Service (ILDIS)
- World Legume Species Checklist at Legumes Online
- Fabaceae at Flowers in Israel
- Asociación Española de las Leguminosas (AEL). Charity founded to promote the agricultural use of legumes in Spain.
- World list of Fabaceae species species (20,856 spp) in the Catalogue of Life