Adaptation (eye)
.jpg)
In ocular physiology, adaptation is the ability of the eye to adjust to various levels of darkness and light.
Efficiency
The human eye can function from very dark to very bright levels of light; its sensing capabilities reach across nine orders of magnitude. This means that the brightest and the darkest light signal that the eye can sense are a factor of roughly 1,000,000,000 apart. However, in any given moment of time, the eye can only sense a contrast ratio of 1,000. What enables the wider reach is that the eye adapts its definition of what is black.
The eye takes approximately 20–30 minutes to fully adapt from bright sunlight to complete darkness and become 10,000 to 1,000,000 times more sensitive than at full daylight. In this process, the eye's perception of color changes as well (this is called the Purkinje effect). However, it takes approximately five minutes for the eye to adapt from darkness to bright sunlight. This is due to cones obtaining more sensitivity when first entering the dark for the first five minutes but the rods take over after five or more minutes.[1]
Dark adaptation is far quicker and deeper in young people than the elderly.[2]
Ambient light response

A minor mechanism of adaptation is the pupillary light reflex, adjusting the amount of light that reaches the retina.
In response to varying ambient light levels, rods and cones of eye function both in isolation and in tandem to adjust the visual system. Changes in the sensitivity of rods and cones in the eye are the major contributors to dark adaptation.
Above a certain luminance level (about 0.03 cd/m2), the cone mechanism is involved in mediating vision; photopic vision. Below this level, the rod mechanism comes into play providing scotopic (night) vision. The range where two mechanisms are working together is called the mesopic range, as there is not an abrupt transition between the two mechanism. This adaptation forms the basis of the Duplicity Theory.[3]
Dark adaptation
Rhodopsin, a biological pigment in the photoreceptors of the retina, immediately photobleaches in response to light.[4] Visual Phototransduction starts with the isomerizing of the pigment chromophore from 11-cis to all-trans retinal.[5] Then this pigment dissociates into free opsin and all-trans retinal. Dark adaptation of both rods and cones requires the regeneration of the visual pigment from opsin and 11-cis retinal.[5]Therefore, the time required for dark adaptation and pigment regeneration is largely determined by the local concentration of 11-cis retinal and the rate at which it is delivered to the opsin in the bleached rods.[6]The decrease in calcium ion influx after channel closing causes phosphorylation of metarhodopsin II and speeds up the cis-retinal to trans-retinal inactivation.[5] The phosphorylation of activated rhodopsin is mediated by recoverin.[5] Rods are more sensitive to light and so take longer to fully adapt to the change in light. Rods, whose photopigments regenerate more slowly, do not reach their maximum sensitivity for about half an hour.[1][7] Cones take approximately 9–10 minutes to adapt to the dark.[1] Sensitivity to light is modulated by changes in intracellular calcium ions and cyclic guanosine monophosphate.[8]
The sensitivity of the rod pathway improves considerably within 5–10 minutes in the dark. Color testing has been used to determine the time at which rod mechanism takes over; when the rod mechanism takes over colored spots appear colorless as only cone pathways encode color.[9]
Mechanism Using Second Messenger Pathway
Normally, calcium reduces the affinity of channels to cGMP, through calcium-binding protein, calmodulin.[10] A decrease in calcium levels when cGMP gated Na+1 channels close activates guanalyl cyclase, which increases production of cGMP, and also increases the affinity of the channels to cGMP to potentiate re-opening of the Na+1 channels.[10] The decrease in calcium ion concentration also inhibits the activation of phosphodiesterase to slow cGMP hydrolysis and increase the amount of cGMP.[10]This allows for the photoreceptor cell to hyperpolarize again in response to changes in brightness level even in the dark because channels would re-open and allow for the cell to slightly depolarize.
Four factors affect dark adaptation:
- Intensity and duration of the pre-adapting light
- By increasing the levels of pre-adapting luminances, the duration of cone mechanism dominance extends, while the rod mechanism switch over is more delayed. In addition the absolute threshold takes longer to reach. The opposite is true for decreasing the levels of pre-adapting luminances.[11]
- Size and location on the retina
- The location of the test spot affects the dark adaptation curve because of the distribution of the rods and cones in the retina.[12]
- Wavelength of the threshold light
- Varying the wavelengths of stimuli also affect the dark adaptation curve. Long wavelengths, such as extreme red, create the absence of a distinct rod/cone break as the rod and cone cells have similar sensitivities to light of long wavelengths. Conversely at short wavelengths the rod/cone break is more prominent, because the rod cells are much more sensitive than cones once the rods have dark adapted.[11]
- Rhodopsin regeneration
- Dark adaptation depends upon photopigment bleaching, which affects the threshold of both cone and rod cells.[11]
Inhibition
Inhibition by neurons also affects activation in synapses. Together with the bleaching of a rod or cone pigment, merging of signals on ganglion cells are inhibited, reducing convergence. Alpha adaptation, i.e., rapid sensitivity fluctuations, is powered by nerve control. The merging of signals by virtue of the diffuse ganglion cells, as well as horizontal and amacrine cells, allow a cumulative effect. Thus that area of stimulation is inversely proportional to intensity of light, a strong stimulus of 100 rods equivalent to a weak stimulus of 1,000 rods.
In sufficiently bright light, convergence is low, but during dark adaptation, convergence of rod signals boost. This is not due to structural changes, but by a possible shutdown of inhibition that stops convergence of messages in bright light. If only one eye is open, the closed eye must adapt separately upon reopening to match the already adapted eye.[1]
Measuring Dark Adaptation
Ophthalmologists sometimes measure patients’ dark adaptation using an instrument known as a dark adaptometer. Currently, there is one commercially available dark adaptometer, called the AdaptDx. It works by measuring a patient’s Rod Intercept (RI) time. RI is the number of minutes it takes for the eye to adapt from bright light to darkness. This RI number provides a clear and objective measurement of retinal function with 90% sensitivity and specificity.[13] Basically, an RI of less than 6.5 minutes indicates a healthy dark adaptation function. However, an RI higher than 6.5 indicates impaired dark adaptation.
Using Dark Adaptation Measurement to Diagnose Disease
Numerous clinical studies have shown that dark adaptation function is dramatically impaired from the earliest stages of AMD, retinitis pigmentosa (RP), and other retinal diseases, with increasing impairment as the diseases progress.[14][15] AMD is a chronic, progressive disease that causes a part of your retina, called the macula, to slowly deteriorate as you get older. It is also the leading cause of vision loss among people age 50 and older.[16] It is characterized by a breakdown of the RPE/Bruch’s membrane complex in the retina, leading to an accumulation of cholesterol deposits in the macula. Eventually, these deposits become clinically-visible drusen that affect photoreceptor health, causing inflammation and a predisposition to choroidal neovascularization (CNV). During the AMD disease course, the RPE/Bruch’s function continues to deteriorate, hampering nutrient and oxygen transport to the rod and cone photoreceptors. As a side effect of this process, the photoreceptors exhibit impaired dark adaptation because they require these nutrients for replenishment of photopigments and clearance of opsin to regain scotopic sensitivity after light exposure.
Measurement of a patient’s dark adaptation function is essentially a bioassay of the health of their Bruch’s membrane. As such, research has shown that, with the AdaptDx, doctors can detect subclinical AMD at least three years earlier than it is clinically evident.[17]
Light adaptation
With light adaptation, the eye has to quickly adapt to the background illumination to be able to distinguish objects in this background. The process for light adaptation occurs over a period of five minutes.
The photochemical reaction is:
- Rhodopsin ⇌ retinal + opsin
Increment threshold
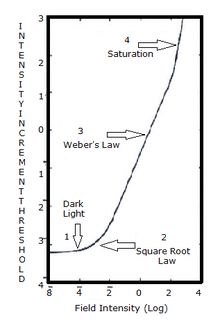
Using increment threshold experiments, light adaptation can be measured clinically.[18] In an increment threshold experiment, a test stimulus is presented on a background of a certain luminance, the stimulus is increased until the detection threshold is reached against the background. A monophasic or biphasic threshold versus intensity TVI curve is obtained through this method for both cones and rods.
When the threshold curve for a single system (i.e., just cones or just rods) is taken in isolation it can been seen to possesses four sections:[19]
- 1. Dark light
- The threshold in this portion of the TVI curve is determined by the dark/light level. Sensitivity is limited by neural noise. The background field is relatively low and does not significantly affect threshold.
- 2. Square root law
- This part of the curve is limited by quantal fluctuation in the background. The visual system is usually compared with a theoretical construct called the ideal light detector. To detect the stimulus, the stimulus must sufficiently exceed the fluctuations of the background (noise).
- 3. Weber's law
- Threshold increases with background luminance proportional to the square root of the background.[20]
- 4. Saturation
- At saturation, the rod system becomes unable to detect the stimulus. This section of the curve occurs for the cone mechanism under high background levels.[21]
Insufficiency
Insufficiency of adaptation most commonly presents as insufficient adaptation to dark environment, called night blindness or nyctalopia. The opposite problem, known as hemeralopia, that is, inability to see clearly in bright light, is much rarer.
The fovea is blind to dim light (due to its cone-only array) and the rods are more sensitive, so a dim star on a moonless night must be viewed from the side, so it stimulates the rods. This is not due to pupil width since an artificial fixed-width pupil gives the same results.[1]
See also
- Accelerating dark adaptation in humans
- Accommodation (eye)
- Adaptive system
- Dark adaptor goggles
- Mesopic vision
- Neural adaptation
References
- 1 2 3 4 5 "Sensory Reception: Human Vision: Structure and Function of the Human Eye" Encyclopædia Britannica, vol. 27, 1987
- ↑ Jackson GR, Owsley C, McGwin G Jr (1999). "Aging and dark adaptation". Vision Res. 39 (23): 3975–82. PMID 10748929. doi:10.1016/s0042-6989(99)00092-9.
- ↑ "Light and Dark Adaptation by Michael Kalloniatis and Charles Luu – Webvision". webvision.med.utah.edu.
- ↑ Stuart JA, Brige RR (1996). "Characterization of the primary photochemical events in bacteriorhodopsin and rhodopsin". In Lee AG. Rhodopsin and G-Protein Linked Receptors, Part A (Vol 2, 1996) (2 Vol Set). Greenwich, Conn: JAI Press. pp. 33–140. ISBN 1-55938-659-2.
- 1 2 3 4 Bhatia, K; Jenkins, C; Prasad, M; Koki, G; Lombange, J (1989). "Immunogenetic studies of two recently contacted populations from Papua New Guinea". Human biology. 61 (1): 45–64. PMID 2707787.
- ↑ Lamb, T. D.; Pugh Jr, E. N. (2004). "Dark adaptation and the retinoid cycle of vision". Progress in Retinal and Eye Research. 23 (3): 307–80. PMID 15177205. doi:10.1016/j.preteyeres.2004.03.001.
- ↑ Passer and Smith (2008). Psychology: The Science of Mind and Behavior (4th ed.). p. 135. ISBN 0-07-256334-6.
- ↑ Hurley, JB (February 2002). "Shedding Light on Adaptation". Journal of General Physiology. 119 (2): 125–128. PMC 2233798
 . PMID 11815663. doi:10.1085/jgp.119.2.125.
. PMID 11815663. doi:10.1085/jgp.119.2.125. - ↑ Aubert H. Physiologie der Netzhaut. Breslau: E. Morgenstern; 1865.
- 1 2 3 Pugh Jr, E. N.; Lamb, T. D. (1990). "Cyclic GMP and calcium: The internal messengers of excitation and adaptation in vertebrate photoreceptors". Vision research. 30 (12): 1923–48. PMID 1962979.
- 1 2 3 Bartlett NR. Dark and light adaptation. In: Graham CH, editor. Vision and visual perception. New York: John Wiley and Sons, Inc.; 1965.
- ↑ Hallett PE. The variations in visual threshold measurement. J Physiol 1969;202:403–419.1351489 [PubMed: 5784294]
- ↑ Jackson, GR (2014). "Diagnostic Sensitivity and Specificity of Dark Adaptometry for Detection of Age-Related Macular Degeneration". Invest Ophthalmol Vis Sci. 55 (3): 1427–1431. PMC 3954002
 . PMID 24550363. doi:10.1167/iovs.13-13745.
. PMID 24550363. doi:10.1167/iovs.13-13745. - ↑ Owsley, C.; Jackson, G. R.; White, M.; Feist, R.; Edwards, D. (2001-07-01). "Delays in rod-mediated dark adaptation in early age-related maculopathy". Ophthalmology. 108 (7): 1196–1202. ISSN 0161-6420. PMID 11425675. doi:10.1016/s0161-6420(01)00580-2.
- ↑ Curcio, CA (2013). Structure, function, and pathology of Bruch’s membrane. In: Ryan SJ, et al, eds. Retina, Vol 1, Part 2: Basic Science and Translation to Therapy. 5th ed. Elsevier.
- ↑ NEI. ""Facts About Age-Related Macular Degeneration"". NEI.
- ↑ Owsley, Cynthia; McGwin, Gerald; Clark, Mark E.; Jackson, Gregory R.; Callahan, Michael A.; Kline, Lanning B.; Witherspoon, C. Douglas; Curcio, Christine A. (2016-02-01). "Delayed Rod-Mediated Dark Adaptation Is a Functional Biomarker for Incident Early Age-Related Macular Degeneration". Ophthalmology. 123 (2): 344–351. ISSN 1549-4713. PMC 4724453
 . PMID 26522707. doi:10.1016/j.ophtha.2015.09.041.
. PMID 26522707. doi:10.1016/j.ophtha.2015.09.041. - ↑ H Davson. Physiology of the eye. 5th ed. London: Macmillan Academic and Professional Ltd.; 1990.
- ↑ Aguilar M, Stiles WS. Saturation of the rod mechanism of the retina at high levels of stimulation. Opt Acta (Lond) 1954;1:59–65.
- ↑ Barlow, H. B. (1958). "Temporal and spatial summation in human vision at different background intensities". The Journal of physiology. 141 (2): 337–350. PMC 1358805
 . PMID 13539843.
. PMID 13539843. - ↑ H Davson. Physiology of the eye. 5th ed. London: Macmillan Academic and Professional Ltd.; 1990
External links
- Adaptation, Ocular at the US National Library of Medicine Medical Subject Headings (MeSH)
- http://webvision.med.utah.edu/light_dark.html