Extracellular fluid
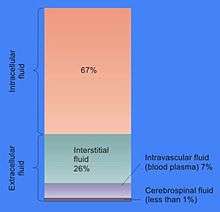
Extracellular fluid (ECF) or extracellular fluid volume (ECFV) usually denotes all body fluid outside the cells. The remainder is called intracellular fluid (ICF). The ECF and ICF are the two major fluid compartments, which together account for total body water (TBW).
In some animals, including mammals, the ECF can be divided into two major subcompartments, interstitial fluid and blood plasma, which make up at least 97%. The extracellular fluid also includes the transcellular fluid, which comprises about 2.5%. It also includes the acellular portion of lymph by the obligate logic of the outside-the-cells definition, although discussions of ECF usually treat lymph as negligible or implicitly lump it together with the interstitial fluid. One way of viewing the ECF is that it has "two components: plasma and lymph as a delivery system, and interstitial fluid for solute exchange."[1]
The interstitial portion of the extracellular fluid constitutes the milieu intérieur or "internal environment" which bathes the all of the body's cells. The ECF composition is therefore crucial for their normal functions. This constancy of the "internal environment" is achieved by means of a set of homeostats or negative feedback systems, which keep the pH, and the sodium (Na+), potassium (K+), and calcium (Ca2+) ion concentrations within very tight limits. The osmolality and glucose concentrations are similarly rigidly regulated, each within a very narrow range of values, as are the partial pressures of oxygen and carbon dioxide.
The volume of ECF is typically 15 L, of which 12 L is interstitial fluid and 3 L is plasma. Interstitial fluid makes up 16% of human body weight, and blood plasma, 4%.
Function
The function of the extracellular fluid is firstly to provide the watery environment in which the cells of the body live and function.[3] The body's cells extract everything they need for their energy and structural metabolism from the ECF, and secrete any wastes and everything that they use to communicate with other cells, into it. The composition of this watery environment is therefore very precisely regulated, especially with regard to its ionic composition (which includes the fluid's pH), the partial pressures of oxygen and carbon dioxide, and several of the high energy nutrients the cells require for their metabolism (e.g. glucose and fatty acids).
There is a significant difference between the concentrations of sodium and potassium ions inside and outside the cell. The concentration of sodium ions is considerably higher in the extracellular fluid than in the intracellular fluid.[3] The converse is true of the potassium ion concentrations inside and outside the cell. These differences cause the cell membranes of all the living cells in the body to be electrically charged, with the positive charge on the outside of the cells and the negative charge on the inside. The electrical potential difference between the two is about 70 mV in a resting cell.[3] This potential difference is created by ionic pumps in the membranes of cells, which pump sodium ions out of the cells, into the ECF, in return for potassium ions which enter the cell from the ECF. In several cell types special ion channels in the cell membrane can be temporarily opened under specific circumstances for a few microseconds at a time. This allows a brief inflow of sodium ions into the cell (driven in by the sodium ion concentration gradient that exists between the outside and inside of the cell). This causes the cell membrane to temporarily depolarize (i.e. lose its electrical charge) forming the basis of ‘’nerve impulses” (also known as action potentials). These action potentials are important means of communication along nerve fibers as well as along muscle fibres, both in the heart and skeletal muscle, and in several other tissues.[3] These events can only occur if the concentrations of sodium and potassium ions in the ECF are at their correct (regulated) values.
The sodium ions in the ECF also play an important role in the movement of water from one body compartment to the other. When tears are secreted, or saliva is formed, sodium ions are pumped from the ECF into the ducts in which these fluids are formed and collected. The water content of these solutions results from the fact water follows the sodium ions (and accompanying anions) osmotically.[3] The same principle applies to the formation of a great many watery secretions in the body.
Calcium ions have a great propensity to bind to proteins.[4] This changes the distribution of electrical charges on the protein, with the consequence that the 3D (or tertiary) structure of the protein is altered.[3] The normal shape, and therefore function of very many of the extracellular proteins, as well as the extracellular portions of the cell membrane proteins is dependent on a very precise ionized calcium concentration in the ECF. The proteins that are particularly sensitive to changes in the ECF ionized calcium concentration are several of the clotting factors in the blood plasma, which are functionless in the absence of calcium ions, but become fully functional on the addition of the correct concentration of calcium salts.[3][4] The voltage gated sodium ion channels in the cell membranes of nerves and muscle have an even greater sensitivity to changes in the ECF ionized calcium concentration.[5] Relatively small decreases in the plasma ionized calcium levels (hypocalcemia) cause these channels to leak sodium into the nerve cells or axons, making them hyper-excitable, thus causing spontaneous muscle spasms (tetany) and paraesthesia (the sensation of "pins and needles") of the extremities and round the mouth.[6] When the plasma ionized calcium rises above normal (hypercalcemia) more calcium is bound to these sodium channels having the opposite effect, causing lethargy, muscle weakness, anorexia, constipation and labile emotions.[6]
The tertiary structure of proteins is also affected by the pH of the bathing solution. In addition, the pH of the ECF affects the proportion of the total amount of calcium in the plasma which occurs in the free, or ionized form, as opposed to the fraction that is bound to protein and phosphate ions. A change in the pH of the ECF therefore alters the ionized calcium concentration of the ECF. Since the pH of the ECF is directly dependent on the partial pressure of carbon dioxide in the ECF, hyperventilation, which lowers the partial pressure of carbon dioxide in the ECF, produces symptoms that are almost indistinguishable from low plasma ionized calcium concentrations.
The extracellular fluid is constantly “stirred” by the circulatory system, which ensures that the watery environment which bathes the body’s cells is virtually identical throughout the body. This means that nutrients can be secreted into the ECF in one place (e.g. the gut, liver, or fat cells) and will, within about a minute, be evenly distributed throughout the body. Hormones are similarly rapidly and evenly spread to every cell in the body, regardless of where they are secreted into the blood. Oxygen taken up by the lungs from the alveolar air is also evenly distributed at the correct partial pressure to all the cells of the body. Waste products are also uniformly spread to the whole of the ECF, and are removed from this general circulation at specific points (or organs), once again ensuring that there is generally no localized accumulation of unwanted compounds or excesses of otherwise essential substances (e.g. sodium ions, or any of the other constituents of the ECF). The only significant exception to this general principle is the plasma in the veins, where the concentrations of dissolved substances in individual veins differs, to varying degrees, from those in the rest of the ECF. However this plasma is confined within the waterproof walls of the venous tubes, and therefore does not affect the interstitial fluid that forms the "pond" in which the body's cell live. When the blood from all the veins in body mixes in the heart and lungs, the differing compositions cancel out (e.g. acidic blood from active muscles is neutralized by the alkaline blood homeostatically produced by the kidneys). From the left atrium onward, to every organ in the body, the normal, homeostatically regulated values of all of the ECF’s components are therefore restored.
Interaction between the blood plasma, interstitial fluid and lymph
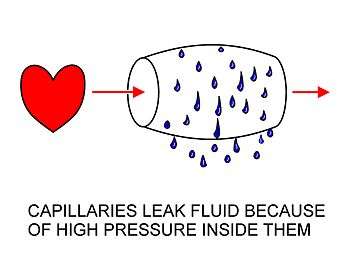
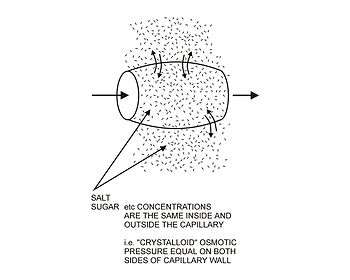
The arterial blood plasma, interstitial fluid and lymph interact at the level of the blood capillaries. These microscopic vessels are not waterproof so water can move freely in and out of them. At the arteriolar end of the capillary the blood pressure, though considerably lower than in the aorta, is still greater than the hydrostatic pressure in the tissues.[2][3] Water will therefore seep out of the capillary into the interstitial fluid. The pores through which this water moves are large enough to allow all the smaller molecules (up to the size of small proteins such as insulin) to move freely through the capillary wall as well. This means that their concentrations across the capillary wall equalize, and therefore have no osmotic effect (because the osmotic pressure caused by these small molecules and ions – called the crystalloid osmotic pressure to distinguish it from the osmotic effect of the larger molecules than cannot move across the capillary membrane – is the same on both sides of capillary wall).[2][3]
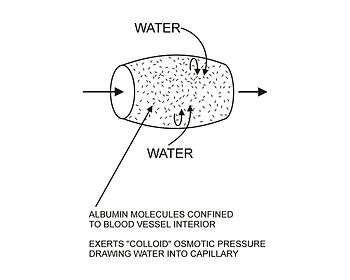
The movement of water out of the capillary at the arteriolar end causes the concentration of the substances that cannot cross the capillary wall to increase as the blood moves to the venular end of the capillary. The most important substances that are confined to the capillary tube are plasma albumin, the plasma globulins and fibrinogen. They, and particularly the plasma albumin, because of its molecular abundance in the plasma, are responsible for the so called ”oncotic” or "colloid" osmotic pressure which draws water back into the capillary, especially at the venular end.[2] (See the diagrams on the left.)
The net effect of all of these processes is that water moves out of and back into the capillary, while the crystalloid substances in the capillary and interstitial fluids equilibrate. Since the capillary fluid is constantly and rapidly renewed by the flow of the blood, its composition dominates the equilibrium concentration that is achieved in the capillary bed. This ensures that the watery environment of the body’s cells is always close to their ideal environment (set by the body’s homeostats).
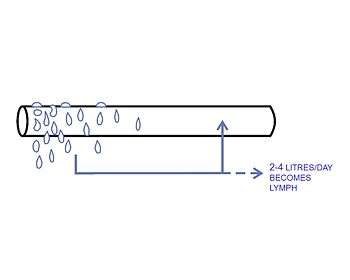
A small proportion of the solution that leaks out of the capillaries is not drawn back into the capillary by the colloid osmotic forces. This amounts to between 2-4 liters per day for the body as a whole. This water is collected by the lymphatic system and is ultimately discharged into the left subclavian vein, where it mixes with the venous blood coming from the left arm, on its way to the heart.[3] The lymph flows through lymph capillaries to lymph nodes where bacteria and tissue debris are removed from the lymph, while various types white blood cells (mainly lymphocytes) are added to the fluid. In addition the lymph which drains the small intestine contains fat droplets called chylomicrons after the ingestion of a fatty meal.[4] This gives this lymph a milky appearance, and imparts the name lacteals (referring to “milk”) to the lymph vessels of the small intestine.[3]
Electrolytic constituents
- Chloride (Cl− = 99–110 mM)
- Bicarbonate (HCO3− = 22–28 mM)
- Phosphate (HPO42− = 0.8-1.4 mmM)
Description
The interstitial fluid component of the ECF is an almost colorless solution, which is salty to the taste. The plasma, which contains the plasma proteins which are largely absent from the interstitial fluid is generally straw colored. It also tastes salty, and has a "sticky" feel due its protein content. Plasma will clot if left standing for more than a few minutes, due to the presence of clotting factors, which are activated by contact with any wettable surface such as the glass of a test tube or simply by stagnation (through an unknown mechanism). Lymph can be almost colorless, but tends to have a slightly turbid appearance due to its content of cells (mainly white blood cells) or fat globules when it drains the intestines after a meal.
Location
Human ECF is found in blood plasma, lymph, body cavities lined with serous (moisture-exuding) membranes, the cavities and channels of the brain and spinal cord, and as interstitial fluid between the cells of all the other tissues of the body.
See also
References
- ↑ Canavan, A; Arant, BS Jr (2009), "Diagnosis and management of dehydration in children" (PDF), Am Fam Physician, 80 (7): 692–696, PMID 19817339.
- 1 2 3 4 5 6 7 Guyton, Arthur; Hall, John (2006). "Chapter 16: The Microcirculation and the Lymphatic System". In Gruliow, Rebecca. Textbook of Medical Physiology (Book) (11th ed.). Philadelphia, Pennsylvania: Elsevier Inc. pp. 187–188. ISBN 0-7216-0240-1.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 Tortora, Gerard J.; Anagnostakos, Nicholas P. (1987). Principles of Anatomy and Physiology (Fifth ed.). New York: Harper & Row, Publishers. pp. 40, 49–50, 61, 268–274, 449–453, 456, 494–496, 530–552, 693–700. ISBN 0-06-350729-3.
- 1 2 3 Stryer, Lubert (1995). Biochemistry. (Fourth ed.). New York: W.H. Freeman and Company. pp. 255–256, 347–348, 697–698. ISBN 0 7167 2009 4.
- ↑ Armstrong CM, Cota G (Mar 1999). "Calcium block of Na+ channels and its effect on closing rate". Proceedings of the National Academy of Sciences of the United States of America. 96 (7): 4154–7. Bibcode:1999PNAS...96.4154A. PMC 22436
 . PMID 10097179. doi:10.1073/pnas.96.7.4154.
. PMID 10097179. doi:10.1073/pnas.96.7.4154. - 1 2 Harrison TR. Principles of Internal Medicine (third ed.). New York: McGraw-Hill Book Company. pp. 170, 571–579.
- 1 2 Diem, K.; Lentner, C. (1970). "Blood – Inorganic substances". in: Scientific Tables (Seventh ed.). Basle, Switzerland: CIBA-GEIGY Ltd. pp. 561–568.