Leech
| Leech Temporal range: Silurian–Recent | |
|---|---|
| | |
| Hirudo medicinalis | |
 | |
| Helobdella sp. | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Annelida |
| Class: | Clitellata |
| Subclass: | Hirudinea Lamarck, 1818 |
| Infraclasses | |
|
Acanthobdellidea | |
Leeches are segmented worms that belong to the phylum Annelida and comprise the subclass Hirudinea.[1] Like the oligochaetes, such as earthworms, leeches share a clitellum and are hermaphrodites. Nevertheless, they differ from the oligochaetes in significant ways. For example, leeches do not have bristles and the external segmentation of their bodies does not correspond with the internal segmentation of their organs. Their bodies are much more solid as the spaces in their coelom are dense with connective tissues. They also have two suckers, one at each end.
The majority of leeches live in freshwater environments, while some species can be found in terrestrial[2] and marine environments. The best-known leeches, such as the medicinal leech, Hirudo medicinalis, are hematophagous, feeding on vertebrate blood and invertebrate hemolymph.[3] Most leech species, however, are predatory, feeding primarily by swallowing other invertebrates. Almost 700 species of leeches are currently recognized, of which some 100 are marine, 90 terrestrial and the remainder freshwater taxa.[4]
Leeches, such as the Hirudo medicinalis, have been historically used in medicine to remove blood from patients.[5] The practice of leeching can be traced to ancient India and Greece, and continued well into the 18th and 19th centuries in both Europe and North America. In modern times, leeches are used medically in procedures such as the reattachment of body parts and reconstructive and plastic surgeries[6] and, in Germany, treating osteoarthritis.[7][8]
Taxonomy and systematics

Leeches are presumed to have evolved from certain Oligochaeta, most of which feed on detritus. However, some species in the Lumbriculidae are predatory and have similar adaptations as found in leeches. As a consequence, the systematics and taxonomy of leeches is in need of review. While leeches form a clade, the remaining oligochaetes are not their sister taxon, but in a diverse paraphyletic group containing some lineages that are closely related to leeches, and others that are far more distant.
There is some dispute as to whether Hirudinea should be a class itself, or a subclass of the Clitellata. The resolution mainly depends on the eventual fate of the oligochaetes, which as noted above, do not form a natural group as traditionally circumscribed. Another possibility would be to include the leeches in the taxon Oligochaeta, which would then be ranked as a class and contain most of the clitellates. The Branchiobdellida are leechlike clitellates that were formerly included in the Hirudinea, but are just really close relatives.
The more primitive Acanthobdellidea are often included with the leeches, but some authors treat them as a separate clitellate group. True leeches of the infraclass Euhirudinea have both anterior and posterior suckers. They are divided into two groups: Arhynchobdellida and Rhynchobdellida
- Rhynchobdellida are "jawless" leeches, armed with a muscular, straw-like proboscis puncturing organ in a retractable sheath. The Rhynchobdellae consist of two families:
- Glossiphoniidae are flattened leeches with poorly defined anterior suckers.
- Piscicolida have cylindrical bodies and usually well-marked, bell-shaped, anterior suckers. The Glossiphoniidae live in freshwater habitats; the Pisciolidae are found in seawater habitats.
- Arhynchobdellida lack a proboscis and may or may not have jaws armed with teeth. Arhynchobellids are divided into two orders:
- Gnathobdela: This order of "jawed" leeches, armed with teeth, includes the quintessential leech: the European medical (bloodsucking) leech, Hirudo medicinalis. It has a tripartite jaw filled with hundreds of tiny, sharp teeth. The incision mark left on the skin by the European medical leech is an inverted Y inside a circle. Its North American counterpart is Macrobdela decora, a much less efficient medical leech.[9] Within this order, the family Hirudidae is characterized by aquatic leeches and the family Haemadipsidae by terrestrial leeches. In the latter are Haemadipsa sylvestris, the Indian leech and Haemadipsa zeylanica (yamabiru), the Japanese mountain or land leech.
- Pharyngobdella: These so-called worm-leeches consist of freshwater or amphibious leeches that have lost the ability to penetrate a host's tissue and suck blood. They are carnivorous and equipped with a relatively large, toothless mouth to ingest worms or insect larvae, which are swallowed whole.
The Pharyngobdella have six to eight pairs of eyes, as compared with five pairs in Gnathobdelliform leeches, and include three related families. The Erpobdellidae are some species from freshwater habitats.
Anatomy and physiology
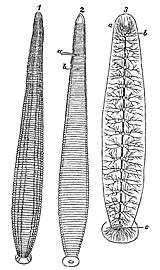

Like other annelids, the leech is a segmented animal. But unlike other annelids, there is no correspondence between the external segmentation of a leech's body surface and the segmentation of its internal organs.[1] The body surface of the animal can be divided into 102 annuli, whereas its internal structures are divided into 32 segments.[10] Of the 32 segments within the body, the first four anterior segments are designated head segments, which include an anterior brain and sucker. These are followed by 21 midbody segments, which include 21 neuronal ganglia, two reproductive organs, and 9 pairs of testes. Finally, the last seven segments are fused to form the animal's tail sucker, as well as its posterior brain.
Reproduction and development
Leeches are hermaphrodites, meaning each has both female and male reproductive organs (ovaries and testes, respectively). Leeches reproduce by reciprocal fertilization, and sperm transfer occurs during copulation. Like earthworms, leeches use a clitellum to hold their eggs and secrete the cocoon.
During reproduction, leeches use hypodermic injection of their sperm. They use a spermatophore, which is a structure containing the sperm. Once next to each other, leeches will line up with one's anterior side opposite the other's posterior. Each leech then shoots the spermatophore into the clitellar region of the other leech, where its sperm will make its way to the female reproductive parts.
The embryonic development of the larva occurs as a series of stages. During stage 1, the first cleavage occurs, which gives rise to an AB and a CD blastomere, and is in the interphase of this cell division when a yolk-free cytoplasm called teloplasm is formed.[11] The teloplasm is known to be a determinant for the specification of the D cell fate.[12] In stage 3, during the second cleavage, an unequal division occurs in the CD blastomere. As a consequence, it creates a large D cell on the left and a smaller C cell to the right. This unequal division process is dependent on actomyosin,[13] and by the end of stage 3 the AB cell divides. On stage 4 of development, the micromeres and teloblast stem cells are formed and subsequently, the D quadrant divides to form the DM and the DNOPQ teloblast precursor cells. By the end stage 6, the zygote contains a set of 25 micromeres, 3 macromeres (A, B and C) and 10 teloblasts derived from the D quadrant.[14]
The teloblasts are pairs of five different types (M, N, O, P, and Q) of embryonic stem cells that form segmented columns of cells (germinal band) in the surface of the embryo.[15] The M-derived cells make mesoderm and some small set of neurons, N results in neural tissues and some ventral ectoderm, Q contributes to the dorsal ectoderm and O and P in the leech are equipotent cells (same developmental potential) that produce lateral ectoderm; however the difference between the two of them is that P creates bigger batches of dorsolateral epidermis than O.[12] The sludgeworm Tubifex, unlike the leech, specifies the O and P lineages early in development and therefore, these two cells are not equipotent.[16] Each segment of the body of the leech is generated from one M, O, P cell types and two N and two Q cells types.[12]
The ectoderm and mesoderm of the body trunk are exclusively derived from the teloblast cells in a region called the posterior progress zone.[17][18] The head of the leech that comes from an unsegmented region, is formed by the first set of micromeres derived from A, B, C and D cells, keeping the bilateral symmetry between the AD and BC cells.[18]
Digestion
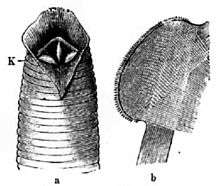
_Cross-section_of_leech_(Hirudinea).jpg)
In most blood-sucking leeches the digestive system starts with the jaws, three blades set at an angle to each other. In feeding they slice their way through the skin of the host, leaving a Y-shaped incision. Behind the blades is the mouth, located ventrally at the anterior end of the body. It leads successively into the pharynx, then the esophagus, the crop, the gizzard, and the intestinum, which ends at the posterior sucker. The crop is a distension of the alimentary canal that functions as an expandable storage compartment. In the crop, some blood-sucking species of leech can store up to five times the body mass of blood. The leech produces an anticoagulant that prevents the stored blood from clotting, plus other agents that inhibit microbial decay of the blood. These measures are so effective that a mature medicinal leech does not need to feed more than twice a year.
The bodies of predatory leeches are similar, though instead of jaws many have a protrusible proboscis, which for most of the time they keep retracted into the mouth. Such leeches often are ambush predators that lie in wait till they can strike prey with the proboscises in a spear-like fashion.[19] Some kinds that live on small invertebrates or detritus have neither proboscis nor jaws, but simply engulf their food with the mouth.
Bacteria in the gut were long thought to carry on digestion for the leech, instead of endogenous enzymes that are very low or absent in the intestine. As discovered relatively recently, all leech species studied do produce endogenous intestinal exopeptidases,[20] which can unlink free terminal-end amino acids, one monomer at a time from a gradually unwinding and degrading protein polymer. However, unzipping of the protein can start from either the amino (tail) or carboxyl (head) terminal-end of the protein molecule. The leech exopeptidases (arylamidases), starting from the tail or amino end and possibly aided by proteases from endosymbiotic bacteria in the intestine, slowly but progressively remove many hundreds of individual terminal amino acids for resynthesis into proteins that constitute the leech. Since leeches lack endopeptidases, the mechanism of protein digestion cannot follow the same sequence as it would in all other animals in which exopeptidases act sequentially on peptides produced by the action of endopeptidases.[20] Exopeptidases are especially prominent in the common North American worm-leech Erpobdella punctata. This evolutionary choice of exopeptic digestion in Hirudinea distinguishes these carnivorous clitellates from Oligochaeta.
Deficiency of digestive enzymes (except exopeptidases), but, more importantly, deficiency of vitamins, B complex for example, in leeches is compensated for by enzymes and vitamins produced by endosymbiotic microflora. In Hirudo medicinalis, these supplementary factors are produced by an obligatory symbiotic relationship with two bacterial species, Aeromonas veronii and a still-uncharacterized Rikenella species. Nonbloodsucking leeches, such as Erpobdella punctata, are host to three bacterial symbionts, Pseudomonas, Aeromonas, and Klebsiella spp. (a slime producer). The bacteria are passed from parent to offspring in the cocoon as it is formed.
Behavior
Leeches are able to display a variety of behaviors that allow them to explore their environments and feed on their hosts. Exploratory behavior includes head movements and body waving.[3]
Feeding
Some leech species prey on small invertebrates, which they swallow whole. To feed on their hosts, leeches use their anterior suckers to connect to hosts for feeding. Once attached, leeches use a combination of mucus and suction to stay attached and secrete an anticoagulant enzyme, hirudin, into the hosts' blood streams. Only certain species of leeches feed on blood, and not all species can bite; 90% of them feed solely on decomposing bodies and open wounds of amphibians, reptiles, waterfowl, fish, and mammals. A leech attaches itself when it bites, and it will stay attached until it becomes full, at which point it falls off to digest. Due to the hirudin secreted, bites may bleed more than a normal wound after the leech is removed.[21]
Leech salivae are commonly believed to contain anesthetic compounds to numb the bite area, but this has never been proven.[22][23] Although morphine-like substances have been found in leeches, they have been found in the neural tissues, not the salivary tissues. They are used by the leeches in modulating their own immunocytes and not for anesthetizing bite areas on their hosts.[23][24] Depending on the species and size, leech bites can be barely noticeable or they can be fairly painful.[23][25][26]
Leeches normally carry parasites in their digestive tracts, which cannot survive in humans and do not pose a threat. However, bacteria, viruses, and parasites from previous blood sources can survive within a leech for months, but only a few cases of leeches transmitting pathogens to humans have been reported.[27] A study found both HIV and hepatitis B in African leeches from Cameroon.[28]
Prevention, removal and treatment
One recommended method of removal is using a fingernail or other flat, blunt object to break the seal of the oral sucker at the anterior end of the leech, repeating with the posterior end, then flicking the leech away. As the fingernail is pushed along the person's skin against the leech, the suction of the sucker's seal is broken, at which point the leech will detach its jaws.[31][32]
Common, but medically inadvisable, techniques to remove a leech are to apply a flame, a lit cigarette, salt, soap, or a chemical such as alcohol, vinegar, lemon juice, insect repellent, heat rub, or certain carbonated drinks. These will cause the leech to quickly detach; however, it will also regurgitate its stomach contents into the wound. The vomit may carry disease, and thus increase the risk of infection.[31][32][33]
An externally attached leech will detach and fall off on its own when it is satiated on blood, which may be anywhere from 20 minutes to two hours or more. After feeding, the leech will detach and depart.[33] Internal attachments, such as inside the nasal passage or vaginal attachments, are more likely to require medical intervention.[34][35]
After removal or detachment, the wound should be cleaned with soap and water, and bandaged. Bleeding may continue for some time, due to the leech's hirudin. Bleeding time will vary, with location, from a few hours to three days. This is a function of the hirudin and other compounds that reduce the surface tension of the blood. Anticlotting medications also affect the bleeding time. Applying pressure can reduce bleeding, although blood loss from a single bite is not dangerous. The wound normally itches as it heals, but should not be scratched, as this may complicate healing and introduce other infections. An antihistamine can reduce itching, and applying a cold pack can reduce pain or swelling.
Some people suffer severe allergic or anaphylactic reactions from leech bites and require urgent medical care. Symptoms include red blotches or an itchy rash over the body, swelling around the lips or eyes, feeling faint or dizzy, and difficulty breathing.[33]
A study conducted by ARPA in 1963 determined that hydroxycitronellal was an effective repellent against both aquatic and terrestrial leeches.[36]
Medicinal use of leeches
The European medical leech Hirudo medicinalis and some congeners, as well as some other species, have been used for clinical bloodletting for thousands of years. The use of leeches in medicine dates as far back as 2,500 years ago, when they were used for bloodletting in ancient India. Leech therapy is explained in ancient Ayurvedic texts. Many ancient civilizations practiced bloodletting, including Indian and Greek civilizations. In ancient Greek history, bloodletting was practiced according to the humoral theory, which proposed that, when the human body's four "humors" — blood, phlegm, and black and yellow bile — were in balance, good health was guaranteed. An imbalance in the proportions of these humors was believed to be the cause of ill health. Records of this theory were found in the Greek philosopher Hippocrates' collection in the fifth century BC. Bloodletting using leeches was one method used by physicians to balance the humors and to rid the body of the plethora.
The use of leeches in modern medicine made a small-scale comeback in the 1980s after years of decline, with the advent of microsurgeries, such as plastic and reconstructive surgeries. In operations such as these, problematic venous congestion can arise due to inefficient venous drainage. Sometimes, because of the technical difficulties in forming an anastomosis of a vein, no attempt is made to reattach a venous supply to a flap at all. This condition is known as venous insufficiency. If this congestion is not cleared up quickly, the blood will clot, arteries that bring the tissues their necessary nourishment will become plugged, and the tissues will die. To prevent this, leeches are applied to a congested flap, and a certain amount of excess blood is consumed before the leech falls away. The wound will also continue to bleed for a while due to the anticoagulant hirudin in the leech's saliva. The combined effect is to reduce the swelling in the tissues and to promote healing by allowing fresh, oxygenated blood to reach the area.[37]
The active anticoagulant component of leech saliva is a small protein, hirudin. Discovery and isolation of this protein led to a method of producing it by recombinant technology. Recombinant hirudin is available to physicians as an intravenous anticoagulant preparation for injection, particularly useful for patients who are allergic to or cannot tolerate heparin.
References
- 1 2 Ralph Buchsbaum; Mildred Buchsbaum; John Pearse; Vicki Pearse (1987). Animals Without Backbone (3rd ed.). Chicago: The University of Chicago Press. pp. 312–317. ISBN 0-226-07874-4.
- ↑ S. Fogden & J. Proctor; Proctor (1985). "Notes on the Feeding of Land Leeches (Haemadipsa zeylanica Moore and H. picta Moore) in Gunung Mulu National Park, Sarawak". Biotropica. 17 (2): 172–174. JSTOR 2388511. doi:10.2307/2388511.
- 1 2 Roy Sawyer (1981). Kenneth, Muller; Nicholls, John; Stent, Gunther, eds. Neurobiology of the Leech. New York: Cold Spring Harbor Laboratory. pp. 7–26. ISBN 0-87969-146-8.
- ↑ Boris Sket; Peter Trontelj (2008). "Global diversity of leeches (Hirudinea) in freshwater". In E. V. Balian; C. Lévêque; H. Segers; K. Martens. Freshwater Animal Diversity Assessment. Hydrobiologia. 595. pp. 129–137. doi:10.1007/s10750-007-9010-8.
- ↑ Brian Payton (1981). Kenneth Muller; John Nicholls; Gunther Stent, eds. Neurobiology of the Leech. New York: Cold Spring Harbor Laboratory. pp. 27–34. ISBN 0-87969-146-8.
- ↑ Adam, Robert; Zakrzewski, Peter (2001). "Therapeutic Use of Leeches: From the 'Annelids' or Medicine". 79 (1). University of Toronto Medical Journal: 65–7.
- ↑ M. Teut & A. Warning; Warning (2008). "Leeches, phytotherapy and physiotherapy in osteo-arthrosis of the knee—a geriatric case study". Forsch Komplementmed. 15 (5): 269–72. PMID 19001824. doi:10.1159/000158875.
- ↑ Michalsen, A; Moebus, S; Spahn, G; Esch, T; Langhorst, J; Dobos, GJ (2002). "Leech therapy for symptomatic treatment of knee osteoarthritis: Results and implications of a pilot study". Alternative therapies in health and medicine. 8 (5): 84–8. PMID 12233807.
- ↑ "freshwater leech". Fcps.edu. Retrieved 2011-11-28.
- ↑ Brian Payton (1981). Kenneth Muller; John Nicholls; Gunther Stent, eds. Neurobiology of the Leech. New York: Cold Spring Harbor Laboratory. pp. 35–50. ISBN 0-87969-146-8.
- ↑ J. Fernandez; N. Olea; V. Tellez; C. Matte (1990). "Structure and development of the egg of the glossiphoniid leech Theromyzon rude: reorganization of the fertilized egg during completion of the first meiotic division". Developmental Biology. 137 (1): 142–154. PMID 2295361. doi:10.1016/0012-1606(90)90015-B.
- 1 2 3 D. A. Weisblat; M. Shankland (1985). "Cell lineage and segmentation in the leech". Philosophical Transactions of the Royal Society B: Biological Sciences. 312 (1153): 39–56. JSTOR 2396301. PMID 2869529. doi:10.1098/rstb.1985.0176.
- ↑ D. C. Lyons; D. A. Weisblat (2009). "D quadrant specification in the leech Helobdella: actomyosin contractility controls the unequal cleavage of the CD blastomere". Developmental Biology. 334 (1): 46–58. PMC 3077801
 . PMID 19607823. doi:10.1016/j.ydbio.2009.07.007.
. PMID 19607823. doi:10.1016/j.ydbio.2009.07.007. - ↑ M. Sandig; W. Dohle (1988). "The cleavage pattern in the leech Theromyzon tessulatum (Hirudinea, Glossiphoniidae)". Journal of Morphology. 196 (2): 217–252. PMID 3385778. doi:10.1002/jmor.1051960210.
- ↑ V. K. Berezovskii; M. Shankland (1996). "Segmental diversification of an identified leech neuron correlates with the segmental domain in which it expresses Lox2, a member of the Hox gene family". Journal of Neurobiology. 29 (3): 319–329. PMID 8907161. doi:10.1002/(SICI)1097-4695(199603)29:3<319::AID-NEU4>3.0.CO;2-C.
- ↑ A. Arai; A. Nakamoto; T. Shimizu (2001). "Specification of ectodermal teloblast lineages in embryos of the oligochaete annelid Tubifex: involvement of novel cell-cell interactions". Development. 128 (7): 1211–1219. PMID 11245587.
- ↑ D. Nardelli-Haefliger; M. Shankland (1993). "Lox10, a member of the NK-2 homeobox gene class, is expressed in a segmental pattern in the endoderm and in the cephalic nervous system of the leech Helobdella". Development. 118 (3): 877–892. PMID 7915671.
- 1 2 M. Shankland; A. E. Bruce (1998). "Axial patterning in the leech: developmental mechanisms and evolutionary implications". Biological Bulletin. 195 (3): 370–372. JSTOR 1543150. PMID 9924777. doi:10.2307/1543150.
- ↑ Fredric R. Govedich; Bonnie A. Bain (March 14, 2005). "All about leeches" (PDF). Retrieved January 19, 2010.
- 1 2 Sawyer, Roy T. "Leech biology and behaviour" (PDF).
- ↑ "Leeches". De Brain. Debrain. Retrieved 21 February 2013.
- ↑ Rigbi Meir; Haim Levy; Amiram Eldor; Fuad Iraqi; Mira Teitelbaum; Miriam Orevi; Amnon Horovitz; Rachel Galun (1987). "The saliva of the medicinal leech Hirudo medicinalis—II. Inhibition of platelet aggregation and of leukocyte activity and examination of reputed anaesthetic effects". Comparative Biochemistry and Physiology C. 88 (1): 95–8. doi:10.1016/0742-8413(87)90052-1.
- 1 2 3 Mark Siddall (July 7, 2008). "Myth Busters: Leech Anaesthetic". Bdellanea. Retrieved December 15, 2013.
- ↑ V. Laurent, B. Salzet, M. Verger-Bocquet, F. Bernet, & M. Salzet; Salzet; Verger-Bocquet; Bernet; Salzet (2000). "Morphine-like substance in leech ganglia. Evidence and immune modulation". European Journal of Biochemistry. 267 (8): 2354–61. PMID 10759861. doi:10.1046/j.1432-1327.2000.01239.x.
- ↑ Mark Siddall; Liz Borda; Gene Burreson; Juli Williams. "Blood Lust II". Laboratory of Phylohirudinology, American Museum of Natural History. Retrieved December 15, 2013.
- ↑ Yi-Te Lai; Jiun-Hong Chen (2010). 臺灣蛭類動物志: Leech Fauna of Taiwan-Biota Taiwanica. 國立臺灣大學出版中心. p. 89. ISBN 9789860227604.
- ↑ A. Ahl-Khleif, M. Roth, C. Menge, J. Heuser, G. Baljer, & W. Herbst; Roth; Menge; Heuser; Baljer; Herbst (2011). "Tenacity of mammalian viruses in the gut of leeches fed with porcine blood". Journal of Medical Microbiology. 60 (6): 787–792. doi:10.1099/jmm.0.027250-0.
- ↑ M. Nehili, C. Ilk, H. Mehlhorn, K. Ruhnau, W. Dick, & M. Njayou; Ilk; Mehlhorn; Ruhnau; Dick; Njayou (1994). "Experiments on the possible role of leeches as vectors of animal and human pathogens: a light and electron microscopy study". Parasitology Research. 80 (4): 277–90. PMID 8073013. doi:10.1007/BF02351867.
- ↑ Don Burke (2005). The complete Burke's backyard: the ultimate book of fact sheets. Murdoch Books. ISBN 1-74045-739-0. Retrieved 2009-09-11.
- ↑ Gary Fujimoto; Marc Robin; Bradford Dessery (2003). The Traveler's Medical Guide. Prairie Smoke Press. ISBN 0-9704482-5-2. Retrieved 2009-09-11.
- 1 2 "The Knowledge: Removing a leech". The Times. October 15, 2006. Retrieved July 28, 2007.
- 1 2 Scenario Archive, Travel Survival: How to Remove a Leech Worst Case Scenarios. Retrieved 2007-07-28. Archived August 25, 2007, at the Wayback Machine.
- 1 2 3 Victorian Poisons Information Centre: Leeches Victorian Poisons Information Centre. Retrieved 2007-07-28.
- ↑ Ibrahim Adibah; Hakim Bilal Gharib; Mohd Nizar (2003). "An Unusual Cause Of Vaginal Bleeding: A Case Report". The Internet Journal of Gynecology and Obstetrics. 2 (2). ISSN 1528-8439.
- ↑ Chow, CK; Wong, SS; Ho, AC; Lau, SK (2005). "Unilateral epistaxis after swimming in a stream". Hong Kong Medical Journal. 11 (2): 110–2. PMID 15815064. Lay summary – Reuters (April 11, 2005).
- ↑ http://www.dtic.mil/dtic/tr/fulltext/u2/413979.pdf
- ↑ http://abcnews.go.com/Health/PainManagement/story?id=4379952
External links
| Wikimedia Commons has media related to Hirudinea. |
| Wikispecies has information related to: Hirudinea |
| The Wikibook Dichotomous Key has a page on the topic of: Hirudinea |
| Wikisource has the text of the 1911 Encyclopædia Britannica article Leech. |
- Leech fact sheet, Australian Museum
- How to remove a leech
- Biotherapy with leeches and maggots
- Leeches in Reconstructive Surgery
- The Science of Leeches, Royal Institution video, August 2013
-
 "Leech". The American Cyclopædia. 1879.
"Leech". The American Cyclopædia. 1879.