Ochronosis
| Ochronosis | |
|---|---|
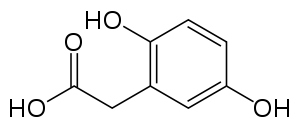 | |
| Homogentisic acid | |
| Classification and external resources | |
| Specialty | endocrinology |
| ICD-10 | E70.2 (ILDS E70.230) |
| ICD-9-CM | 270.2 |
| DiseasesDB | 409 |
| eMedicine | derm/476 |
| MeSH | D009794 |
Ochronosis is a syndrome caused by the accumulation of homogentisic acid in connective tissues. It was first described by Rudolf Virchow in 1865.[1] The condition was named after the yellowish (ocher-like) discoloration of the tissue seen on microscopic examination. However, macroscopically the affected tissues appear bluish grey because of a light-scattering phenomenon known as the Tyndall effect. The condition is most often associated with alkaptonuria but can occur from exogenous administration of phenol complexes like hydroquinone.
Exogenous ochronosis
There are two types of ochronosis: endogenous and exogenous. The endogenous variety is an autosomal recessive disease, that is caused by a lack of homogentisate oxidase enzyme.[2] Exogenous ochronosis is an avoidable dermatitis, that can be caused by the topical application of compounds such as hydroquinone or phenols.[2] It was first seen in 1912, when a patient who used phenol on a leg ulcer was found by Beddard and Plumtre to have this condition.[3] Hydroquinone-induced exogenous ochronosis was found in 1975 by Findlay, who observed the condition in patients who used skin lightening creams containing the compound.[4]
There are three clinical stages of exogenous ochronosis:[5]
- erythema and mild hyperpigmentation
- hypergimentation and "caviar-like" lesions
- papulo-nodular lesions
Causes
Exogenous ochronosis can be caused from long-term usage of certain "skin lightening" products, even if the hydroquinone is in amounts as small as 2%.[2] Skin lightening products are still prevalent in many parts of the world.[6] This may be due to aesthetic or social standing reasons, in areas where a lighter skin tone is considered to be a sign of wealth or beauty.[6] As well, skin-lightening creams containing compounds such as hydroquinone are commonly used to help with hyperpigmentation disorders such as melasma.[7]
Hydroquinone is the compound most frequently used in skin whitening products. Due to concerns about its side effects, it was almost banned by the FDA in 2006, as there were medical issues of carcinogenicity and reports of disfiguring ochronosis.[8] In the European Union hydroquinone has been banned in cosmetic creams since 2000.[9]
Long-term use of creams containing this compound may lead to exogenous ochronotic lesions. The duration of the use is directly proportional to the risk of developing the condition with most cases being after years of use.[2] Around 10–15 million skin lightening products are sold annually, with Japan being the major buyer.[10]
Symptoms
Symptoms include:[11]
- yellow-brown, banana-shaped fibers
- caviar-like papules
- brown-grey or blue-black hyper-pigmentation
The majority of the lesions will be seen on areas of the body that get the most sun.[2]
Treatment
Hydroquinone-induced exogenous ochronosis is an avoidable dermatosis that is exceedingly difficult to treat. However, some studies show that treatment may be possible with a Q-switched alexandrite (755 nm) laser.[12]
It is recommended that individuals with this disorder stop using hydroquinone-containing compounds.[2] It is important to be aware of this as dermatologists may think the symptoms a patient is exhibiting are a melasma, and prescribe a hydroquinone-containing cream.[12]
Pathophysiology
Ochronosis occurs because of deposition of phenols (such as homogentisic acid and hydroquinone) as plaques in the matrix of cartilage. The pigments can also be incorporated into collagen and elastin fibers. In the skin, the pigment alters the structure of the fibers causing enlargement and curling. The embedded pigments also form cross-linkages with pigment depositions in adjacent fibers, stabilizing and reducing the elastic recoil of the fibers. This results in hardening of elastic structures increasing their rigidity and brittleness. Once ruptured, the exposed pigments cause a foreign body reaction and inflammation. This pigment deposition also invokes deposition of hydroxyapatite, the mineral responsible for bone calcification, further hardening the connective tissue. The pigment can also be excreted by glandular cells in apocrine and ceruminous sweat glands as well as breast and prostate tissue. This results in darkly pigmented sweat and breast milk. Excretion of the pigment is only found in endogenous ochronosis and should not occur from topical phenols.

Symptoms
- Skin: The pigment is deposited throughout the skin but only becomes apparent in certain locations where the concentration is great enough to be seen clinically. This usually occurs in areas where connective tissue is thick (joints, tympanic membrane) or close to the surface of the skin (thenar and hypothenar eminences and the sides of the fingers). In exogenous ochronosis, the hyperpigmentation is localized to the area where the inciting agent is applied. Intradermal nevi can appear like blue nevi.
- Eye: The most obvious change is darkening around the palpebral fissure. The cornea can become hyperpigmented if exposed to phenol vapors.
- Cartilage: Darkening and hardening of ear cartilage is a prominent feature of ochronosis. Nasal cartilage is also frequently involved. The voice can be affected by hardening of the laryngeal cartilage. Stiffening of the ribs with decreased lung function has also been reported. The intravertebral cartilage is also more prone to herniation.
- Connective tissue: Hardening of tendons and ligaments can predispose them to rupture. Color changes in the joints can be observed clinically. Arthropathy is common due to chronic inflammation and microruptures.
- Heart valves: Stenosis can results from the increased rigidity of the connective tissue as well as chronic inflammation.[1]
Treatment
Treatment is predominantly preventive. Avoidance of topical phenols and diets low in tyrosine may help. Replacement and repair of damaged tissue is also possible.
See also
References
- 1 2 Findlay GH, et al. Ochronosis. Clinics in Dermatology 1989;7:28-35
- 1 2 3 4 5 6 Charlín, R., Barcaui, C. B., Kac, B. K., Soares, D. B., Rabello-Fonseca, R. and Azulay-Abulafia, L. (2008), Hydroquinone-induced exogenous ochronosis: a report of four cases and usefulness of dermoscopy. International Journal of Dermatology, 47: 19–23. doi:10.1111/j.1365-4632.2007.03351.x
- ↑ Beddard AP, Plumtre CM. "A further note on ochronosis associated with carboluria". Q S Med 1912; 5: 505–507.
- ↑ FINDLAY, G., MORRISON, J. and SIMSON, I. (1975), Exogenous ochronosis and pigmented colloid milium from hydroquinone bleaching creams. British Journal of Dermatology, 93: 613–622. doi:10.1111/j.1365-2133.1975.tb05110.x
- ↑ Dogliotte M, Leibowitz M. "Granulomatous ochronosis – a cosmetic- induced skin disorder in blacks". S Afr Med J 1979; 56: 757–760.
- 1 2 Joan Baxter (18 April 2000). "BBC News - AFRICA - The heavy cost of light skin". BBC.co.uk. Retrieved 9 January 2017.
- ↑ Rajaratnam R, Halpern J, Salim A, Emmett C. Interventions for melasma. Cochrane Database of Systematic Reviews 2010, Issue 7. Art. No.: CD003583. doi:10.1002/14651858.CD003583.pub2.
- ↑ Toombs, E. L. (2007), Hydroquinone – what is it's [sic] future?. Dermatologic Therapy, 20: 149–156. doi:10.1111/j.1529-8019.2007.00128.x
- ↑ "Skin lightening products - The Facts About - CTPA". TheFactsAbout.co.uk. Retrieved 9 January 2017.
- ↑ Leah Armstrong. "Global skin lightening market predicted to reach $10 billion by 2015 – WHITERskin". WhiterSkin.info. Retrieved 9 January 2017.
- ↑ Olumide, Y. M., Akinkugbe, A. O., Altraide, D., Mohammed, T., Ahamefule, N., Ayanlowo, S., Onyekonwu, C. and Essen, N. (2008), Complications of chronic use of skin lightening cosmetics. International Journal of Dermatology, 47: 344–353. doi:10.1111/j.1365-4632.2008.02719.x
- 1 2 Bellew, S. G. and Alster, T. S. (2004), Treatment of Exogenous Ochronosis With a Q-Switched Alexandrite (755 nm) Laser. Dermatologic Surgery, 30: 555–558. doi:10.1111/j.1524-4725.2004.30177.x