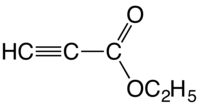
Ethyl propiolate
 | |
| Names | |
|---|---|
| Other names
ethyl propynoate ethyl acetylenecarboxylate | |
| Identifiers | |
| 3D model (JSmol) |
|
| 878250 | |
| ChemSpider | |
| EC Number | 210-795-8 |
| PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C5H6O2 | |
| Molar mass | 98.10 g·mol−1 |
| Appearance | colorless liquid |
| Density | 0.968 g/mL |
| Boiling point | 120 °C (248 °F; 393 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Ethylpropiolate is an organic compound with the formula HC2CO2C2H5. It is the ethyl ester of propiolic acid, the simplest acetylenic carboxylic acid. It is a colorless liquid that is miscible with organic solvents. The compound is a reagent and building block for the synthesis of other organic compounds, reactions that exploit the electrophilicity of the alkyne group.[1]
References
- ↑ Dennis E. Vogel, George H. Büchi (1988). "α-Unsubstituted γ,δ-Unsaturated Aldehydes by Claisen Rearrangement: 3-phenyl-4-pentenal". Org. Synth. 66: 29. doi:10.15227/orgsyn.066.0029.
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.