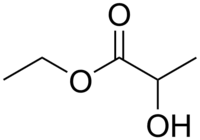
Ethyl lactate
 | |
| Names | |
|---|---|
| IUPAC name
Ethyl 2-hydroxypropanoate | |
| Other names
Ethyl lactate; Lactic acid ethyl ester; 2-Hydroxypropanoic acid ethyl ester; Actylol; Acytol | |
| Identifiers | |
| 3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.010.632 |
| PubChem CID |
|
| RTECS number | OD5075000 |
| |
| |
| Properties | |
| C5H10O3 | |
| Molar mass | 118.13 g·mol−1 |
| Appearance | Clear to slightly yellow liquid |
| Density | 1.03 g/cm3 |
| Melting point | −26 °C (−15 °F; 247 K) |
| Boiling point | 151 to 155 °C (304 to 311 °F; 424 to 428 K) |
| Miscible | |
| Solubility in ethanol and most alcohols |
Miscible |
| Chiral rotation ([α]D) |
−11.3° |
| -72.6·10−6 cm3/mol | |
| Structure | |
| 3.46 D [1] | |
| Hazards | |
| Main hazards | Irritant (Xi) |
| EU classification (DSD) (outdated) |
|
| R-phrases (outdated) | R10 R37 R41 |
| S-phrases (outdated) | (S2) S24 S26 S39 |
| NFPA 704 | |
| Flash point | 46 °C (115 °F; 319 K) |
| Related compounds | |
| Related compounds |
Lactic acid |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Ethyl lactate, also known as lactic acid ethyl ester, is a monobasic ester formed from lactic acid and ethanol, commonly used as a solvent. This compound is considered biodegradable and can be used as a water-rinsible degreaser. Ethyl lactate is found naturally in small quantities in a wide variety of foods including wine, chicken, and various fruits. The odor of ethyl lactate when dilute is mild, buttery, creamy, with hints of fruit and coconut.
Ethyl lactate is produced from biological sources, and can be either the levo (S) form or dextro (R) form, depending on the organism that is the source of the lactic acid. Most biologically sourced ethyl lactate is ethyl (−)-L-lactate (ethyl (S)-lactate). Ethyl lactate is also produced industrially from petrochemical stocks, and this ethyl lactate consists of the racemic mixture of levo and dextro forms. In some jurisdictions, the natural product is exempt from many restrictions placed upon use and disposal of solvents. Because both enantiomers are found in nature, and because ethyl lactate is easily biodegradable, it is considered to be a "green solvent."
Due to its relatively low toxicity, ethyl lactate is used commonly in pharmaceutical preparations, food additives,[2] and fragrances. Ethyl lactate is also used as solvent for nitrocellulose, cellulose acetate, and cellulose ethers.[3]
Ethyl lactate hydrolyzes in the presence of water and acids or bases into lactic acid and ethanol.
Ethyl lactate can be used as a cosolvent to produce suitable conditions for the formation of aryl aldimines.[4]
References
- ↑ Aparicio, Santiago; Alcalde, Rafael (2009). "The green solvent ethyl lactate: an experimental and theoretical characterization". Green Chemistry. 11 (1): 65–78. doi:10.1039/b811909k.
- ↑ U.S. Food and Drug Administration, Center for Food Safety and Applied Nutrition Archived 7 January 2009 at the Wayback Machine.
- ↑ "Industrial Solvents Handbook" by Ernest W. Flick. 5th Edition. William Andrew Inc., 1998. ISBN 0-8155-1413-1, ISBN 978-0-8155-1413-8
- ↑ Jacqueline S. Bennett; Kaitlyn L. Charles; Matthew R. Miner; Caitlin F. Heuberger; Elijah J. Spina; Michael F. Bartels; Taylor Foreman (2009). "Ethyl lactate as a tunable solvent for the synthesis of aryl aldimines". Green Chem. 11 (2): 166–168. doi:10.1039/b817379f.
Further reading
- Pereira, Carla S. M.; Silva, Viviana M. T. M.; Rodrigues, Alírio E. (2011). "Ethyl lactate as a solvent: Properties, applications and production processes – a review". Green Chemistry. 13 (10): 2658. doi:10.1039/C1GC15523G.
