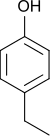
4-Ethylphenol
 | |
| Names | |
|---|---|
| Preferred IUPAC name
4-Ethylphenol | |
| Other names
p-Ethylphenol (no longer recommended[2]) 1-Ethyl-4-hydroxybenzene 1-Hydroxy-4-ethylbenzene 4-hydroxyphenylethane | |
| Identifiers | |
| 3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.004.181 |
| KEGG | |
| UNII | |
| |
| |
| Properties | |
| C8H10O | |
| Molar mass | 122.16 g/mol |
| Appearance | White solid |
| Melting point | 42 to 45 °C (108 to 113 °F; 315 to 318 K) |
| Boiling point | 218 °C (424 °F; 491 K) |
| Hazards | |
| EU classification (DSD) (outdated) |
|
| S-phrases (outdated) | S7/9 S26 S36/37/39 S45 |
| NFPA 704 | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
4-Ethylphenol, often abbreviated to 4-EP, is a phenolic compound.
Natural occurrences
In wine and beer, it is produced by the spoilage yeast Brettanomyces. When it reaches concentrations greater than the sensory threshold (140 µg/L) it can give the wine aromas described as barnyard, medicinal, band-aids, and mousy. In certain Belgian beer styles, a high 4-EP level may be desirable; however, very high levels of the compound in wine can render it undrinkable. The level of 4-ethylphenol is roughly proportional to Brettanomyces concentration and activity, and can therefore be used as an indicator of the yeast's presence. There are significant differences between strains of Brettanomyces in their ability to produce 4-ethylphenol.
It is also a component of castoreum, the exudate from the castor sacs of the mature North American beaver (Castor canadensis) and the European beaver (Castor fiber), used in perfumery.
Biochemistry
4-Ethylphenol is produced from the precursor p-coumaric acid. Brettanomyces converts this to 4-vinylphenol via the enzyme cinnamate decarboxylase.[3] 4-Vinylphenol is further reduced to 4-ethylphenol by the enzyme vinyl phenol reductase. Coumaric acid is sometimes added to microbiological media, enabling the positive identification of Brettanomyces by smell.
 The conversion of p-coumaric acid to 4-ethyphenol by Brettanomyces
The conversion of p-coumaric acid to 4-ethyphenol by Brettanomyces
See also
References
- ↑ 4-Ethylphenol MSDS
- ↑ Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 690. ISBN 978-0-85404-182-4. doi:10.1039/9781849733069-FP001.
Only one name is retained, phenol, for C6H5-OH, both as a preferred name and for general nomenclature. The structure is substitutable at any position. Locants 2, 3, and 4 are recommended, not o, m, and p.
- ↑ Brettanomyces Monitoring by Analysis of 4-ethylphenol and 4-ethylguaiacol Archived 2008-02-19 at the Wayback Machine. at etslabs.com
