Etest
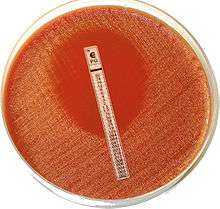
Etest, (previously known as Epsilometer test) manufactured by bioMérieux, is a manual in vitro diagnostic device used by laboratories to determine the MIC (Minimum Inhibitory Concentration) and whether or not a specific strain of bacterium or fungus is susceptible to the action of a specific antimicrobial. This type of test is most commonly used in healthcare settings to help guiding physicians in treatment of patients by indicating what concentration of antimicrobial would successfully treat an infection.
History
The Etest principle was first described in 1988 and was introduced commercially in 1991 by AB BIODISK. AES Chemunex acquired AB BIODISK in 2008 and BioMerieux acquired AES in 2013 continues to manufacture and market this product range under the name Etest.
During the 1950s, Hans Ericsson (Professor of microbiology at the Karolinska Hospital and Karolinska Institute, Stockholm), the scientific founder of AB BIODISK, developed a method to standardize the disc diffusion method and to improve its reproducibility and reliability for clinical susceptibility predictions. The inhibition zone sizes from disc test results were compared to Minimum Inhibitory Concentration (MIC) values based on the reference agar dilution procedure. The correlation between zone sizes and MIC values was then assessed using regression analysis and regression lines were used for extrapolating zone interpretive limits that corresponded to the MIC breakpoint values that defined susceptible, intermediate and resistant categorical results.
Etest was first presented at the Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC) in Los Angeles in 1988 as a novel gradient concept for MIC determinations. In September 1991, Etest was launched globally as a MIC product after receiving the USA Food and Drug Administration (FDA) clearance.
Etest applications include many groups of fastidious organisms, fungi (yeast and mould) and mycobacteria as well as detecting various mechanisms of resistance and MIC testing of key antibiotics with critical specimens e.g. blood and cerebral spinal fluid (CSF).
Intended use
Etest is a quantitative technique for determining the antimicrobial susceptibility (AST) and MIC (in µg/mL) of Gram-negative and Gram-positive aerobic bacteria such as Enterobacteriaceae, Pseudomonas, Staphylococcus, and Enterococcus species and fastidious bacteria, such as anaerobes, N. gonorrhoeae, S. pneumoniae, Streptococcus and Haemophilius species.
Principle
Etest is a ‘ready-to-use’ , inert and non poreus plastic reagent strip with a predefined gradient of antibiotic, covering a continuous concentration range, for the determination of precise MIC values of a wide range of antimicrobial agents against different organism groups.
When Etest is applied to the surface of an agar plate inoculated with the test strain, there is an instantaneous release of the antimicrobial gradient from the plastic carrier to the agar to form a stable and continuous gradient beneath and in the immediate vicinity of the strip.
Etest incubation and reading times have been determined based on the intrinsic growth characteristics of the organism, and the specific incubation conditions. Therefore, for reliable and reproducible results, the stability of the gradient must be maintained for many hours. The predefined Etest gradient remains stable for at least 18 to 24 hours; that is, a period that covers the critical times of many species of fastidious and non-fastidious organisms.
When the Etest strip is placed on an agar surface, the antibiotic gradient on the strip is simply transferred to the agar matrix creating an imprint of the gradient on the strip in the agar. The bacterial growth becomes visible after incubation and a symmetrical inhibition ellipse centered along the strip is seen. The MIC value is read from the scale in terms of µg/mL where the ellipse edge intersects the strip.
Choice of agar medium
Etest can be used with many different kinds of AST agar medium as long as the medium supports good growth of the test organism and does not interfere with the activity of the antimicrobial agent. However, to maximise reproducibility, the medium chosen should fulfil the basic requirements for a susceptibility test medium. The following AST mediums are recommended for use with Etest:
- Aerobes: Mueller Hinton agar such as MHE (bioMérieux)
- Anaerobes: Brucella blood agar with appropriate supplements
These media may require supplemental nutrients to obtain enhanced growth of nutritionally fastidious organisms such as pneumococci, streptococci, Abiotrophia, Haemophilus, gonococci, meningococci and Campylobacter. In general, media recommendations from the CLSI (Clinical and Laboratory Standards Institute) and the EUCAST (European Committee on Antimicrobial Susceptibility Testing) are considered appropriate for Etest.
Result interpretation
After the required incubation period, and only when an even lawn of growth is distinctly visible, the MIC value can be read where the edge of the inhibition ellipse intersects the side of the strip. The plate should not be read if the culture appears mixed or if the lawn of growth is too light or too heavy. Etest MIC endpoints are usually clear-cut although different growth/inhibition patterns may be seen.
Availability
Etest products for more than 100 antimicrobial agents, including antibiotics, antifungal agents and antimycobacterial agents are available. In addition, specific Etest products are available for the detection of specific resistance mechanisms [e.g. ESBL (Extended Spectrum Beta-Lactamase), MBL (Metallo Beta-Lactamase), AmpC Beta-Lactamase and VISA/h VISA].
Etest has been FDA cleared and CE marked for many organisms by comparing to conventional broth/agar dilution reference methods and shown to have excellent correlation. This is a partial list of organisms and antibiotics for which the test has been FDA cleared and CE marked.
Etest equipment
The Etest family of instruments is designed to simplify the daily use of Etest. Simplex C76, Nema C88, and Retro C80 are easy to use, reducing operator fatigue, saving time and improving the quality of results by increasing reproducibility. Etest and related instruments offer one of the most efficient methods for generating on-scale MIC values across 15 doubling dilutions for susceptibility testing of a wide range of drug-bug combinations, including fastidious organisms.
- Simplex C76 automates the placement of 1 to 6 different Etest strips to simplify the setup of MIC panels. Application of up to 6 strips for large agar plates or up to 2 strips on small plates takes <12 seconds.
- Retro C80 is a rota-plater that simplifies and standardizes the inoculation of small and large agar plates making Etest® easier to read when compared to manual streaking.
- Nema C88 is a vacuum pen that simplifies the application of Etest® strips. The applicator is held like a pen and the evacuation hole is covered with the fingertip to create suction. The suction cup is placed on the strip to lift it up and then position onto the agar surface. The strip is released by removing the finger tip from the evacuation hole.
References
Etest has >3,000 scientific references in which it has been tested A few key references are as follows:
- Joyce LF, Downes J, Stockman K, Andrew JH (1 October 1992). "Comparison of five methods, including the PDM Epsilometer test (E test), for antimicrobial susceptibility testing of Pseudomonas aeruginosa". Journal of Clinical Microbiology 30 (10): 2709–2713. PMC 270503. PMID 1400972.
- Goldstein, F.W., et al. 2007. “Comparison of Etest with agar-dilution for testing the susceptibility of P. aeruginosa and other MDR bacteria to Colistin.” JAC. 1039-1040.
- Morosini, M., et al. 2005. “Breakpoints for Predicting P.aeruginosa Susceptibility to Inhaled Tobramycin in Cystic Fibrosis Patients: Use of High-Range Etest strips.” JCM. 43:4480-4485.