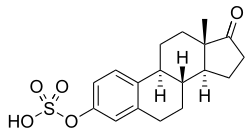
Estrone sulfate
 | |
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral[1] |
| Pharmacokinetic data | |
| Biological half-life | 12 hours[2] |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.006.888 |
| Chemical and physical data | |
| Formula | C18H22O5S |
| Molar mass | 350.43 g/mol |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Estrone sulfate (E1S), or 17β-estrone 3-sulfate, is a natural, endogenous steroid and an estrogen ester and conjugate.[3]
Overview
E1S itself is biologically inactive, with less than 1% of the relative binding affinity of estradiol for the ERα and ERβ,[4] but it can be converted by steroid sulfatase (also called estrogen sulfatase) into estrone, which is an estrogen.[5] Simultaneously, estrogen sulfotransferases convert estrone to E1S, resulting in an equilibrium between the two steroids in various tissues.[5] E1S is thought to serve as a long-lasting reservoir for estrone and estradiol in the body.[6][7] In addition to its biological role, as the sodium salt, sodium estrone sulfate, E1S is the primary active estrogen in conjugated equine estrogens (Premarin) and esterified estrogens.[1] Aside from its presence in these estrogen formulations, E2S is not available as a commercial pharmaceutical drug. However, it is available as estropipate (piperazine estrone sulfate), a salt of E1S and piperazine.[1]
When exogenous estradiol is administered orally, it is subject to extensive first-pass metabolism (95%) in the intestines and liver.[8][9] A single administered dose of estradiol is absorbed 15% as estrone, 25% as estrone sulfate, 25% as estradiol glucuronide, and 25% as estrone glucuronide.[8] Formation of estrogen glucuronide conjugates is particularly important with oral estradiol as the percentage of estrogen glucuronide conjugates in circulation is much higher with oral ingestion than with parenteral estradiol.[8] Estrone glucuronide can be reconverted back into estradiol, and a large circulating pool of estrogen glucuronide and sulfate conjugates serves as a long-lasting reservoir of estradiol that effectively extends its terminal half-life of oral estradiol.[8][9] In demonstration of the importance of first-pass metabolism and the estrogen conjugate reservoir in the pharmacokinetics of estradiol,[8] the terminal half-life of oral estradiol is 13 to 20 hours[10] whereas with intravenous injection its terminal half-life is only about 1 to 2 hours.[11]
See also
- Estradiol sulfate
- Estriol sulfate
- Estriol glucuronide
- DHEA sulfate
- Pregnenolone sulfate
- Lipoidal estradiol
- Catechol estrogen
References
- 1 2 3 Mary C. Brucker; Tekoa L. King (8 September 2015). Pharmacology for Women’s Health. Jones & Bartlett Publishers. pp. 361–. ISBN 978-1-284-05748-5.
- ↑ Lynn Wecker; Stephanie Watts; Carl Faingold; George Dunaway; Lynn Crespo (1 April 2009). Brody's Human Pharmacology. Elsevier Health Sciences. pp. 456–. ISBN 0-323-07575-4.
- ↑ Rogerio A. Lobo (5 June 2007). Treatment of the Postmenopausal Woman: Basic and Clinical Aspects. Academic Press. pp. 768–. ISBN 978-0-08-055309-2.
- ↑ Kuiper GG, Carlsson B, Grandien K, Enmark E, Häggblad J, Nilsson S, Gustafsson JA (1997). "Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta". Endocrinology. 138 (3): 863–70. PMID 9048584. doi:10.1210/endo.138.3.4979.
- 1 2 Tommaso Falcone; William W. Hurd (22 May 2013). Clinical Reproductive Medicine and Surgery: A Practical Guide. Springer Science & Business Media. pp. 5–6. ISBN 978-1-4614-6837-0.
- ↑ Shlomo Melmed; Kenneth S. Polonsky; P. Reed Larsen; Henry M. Kronenberg (11 November 2015). Williams Textbook of Endocrinology (13th ed.). Elsevier Health Sciences. pp. 607–. ISBN 978-0-323-34157-8.
- ↑ James M. Greenblatt; Kelly Brogan (27 April 2016). Integrative Therapies for Depression: Redefining Models for Assessment, Treatment and Prevention. CRC Press. pp. 198–. ISBN 978-1-4987-0230-0.
- 1 2 3 4 5 Michael Oettel; Ekkehard Schillinger (6 December 2012). Estrogens and Antiestrogens II: Pharmacology and Clinical Application of Estrogens and Antiestrogen. Springer Science & Business Media. pp. 268–. ISBN 978-3-642-60107-1.
- 1 2 Christian Lauritzen; John W. W. Studd (22 June 2005). Current Management of the Menopause. CRC Press. pp. 364–. ISBN 978-0-203-48612-2.
- ↑ Stanczyk, Frank Z.; Archer, David F.; Bhavnani, Bhagu R. (2013). "Ethinyl estradiol and 17β-estradiol in combined oral contraceptives: pharmacokinetics, pharmacodynamics and risk assessment". Contraception. 87 (6): 706–727. ISSN 0010-7824. doi:10.1016/j.contraception.2012.12.011.
- ↑ Düsterberg B, Nishino Y (1982). "Pharmacokinetic and pharmacological features of oestradiol valerate". Maturitas. 4 (4): 315–24. PMID 7169965.