Estradiol
 | |
 | |
| Names | |
|---|---|
| Pronunciation | /ˌɛstrəˈdaɪoʊl/ ES-trə-DYE-ohl[1][2] |
| IUPAC name
(8R,9S,13S,14S,17S)-13-Methyl-6,7,8,9,11,12,14,15,16,17-decahydrocyclopenta[a]phenanthrene-3,17-diol | |
| Other names
Estra-1,3,5(10)-triene-3,17β-diol; 17β-Estradiol | |
| Identifiers | |
| 3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.000.022 |
| KEGG | |
| PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C18H24O2 | |
| Molar mass | 272.38 g/mol |
| -186.6·10−6 cm3/mol | |
| Pharmacology | |
| G03CA03 (WHO) | |
| Oral, sublingual, intranasal, topical/transdermal, vaginal, intramuscular or subcutaneous (as an ester), subdermal implant | |
| Pharmacokinetics: | |
| Oral: <5%[3] | |
| ~98%:[3][4] • Albumin: 60% • SHBG: 38% • Free: 2% | |
| Liver (via hydroxylation, sulfation, glucuronidation) | |
| Oral: 13–20 hours[3] Sublingual: 8–18 hours[5] Topical (gel): 36.5 hours[6] | |
| Urine: 54%[3] Feces: 6%[3] | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Estradiol (E2), also spelled oestradiol, is a steroid, an estrogen, and the primary female sex hormone. It is named for and is important in the regulation of the estrous and menstrual female reproductive cycles. Estradiol is essential for the development and maintenance of female reproductive tissues such as the breasts, uterus, and vagina during puberty, adulthood, and pregnancy,[7] but it also has important effects in many other tissues, including bone, fat, skin, liver, and the brain. While estrogen levels in men are lower compared to those in women, estrogens have essential functions in men, as well. It is found in most vertebrates and crustaceans, insects, fish, and other animal species.[8][9]
Estradiol is produced especially within the follicles of the female ovaries, but also in other endocrine (i.e., hormone-producing) and nonendocrine tissues (e.g., including fat, liver, adrenal, breast, and neural tissues). Estradiol is biosynthesized from cholesterol through a series of chemical intermediates.[10] One principal pathway involves the generation of androstenedione, which is converted into estrone by aromatase and then by 17β-hydroxysteroid dehydrogenase into estradiol. Alternatively, androstenedione can be converted into testosterone, an androgen and the primary male sex hormone, which in turn can be aromatized into estradiol.
Biological function
Sexual development
The development of secondary sex characteristics in women is driven by estrogens, to be specific, estradiol.[11][12] These changes are initiated at the time of puberty, most are enhanced during the reproductive years, and become less pronounced with declining estradiol support after menopause. Thus, estradiol produces breast development, and is responsible for changes in the body shape, affecting bones, joints, and fat deposition.[11][12] In females, estradiol induces breast development, widening of the hips, a feminine fat distribution (with fat deposited particularly in the breasts, hips, thighs, and buttocks), and maturation of the vagina and vulva, whereas it mediates the pubertal growth spurt (indirectly via increased growth hormone secretion)[13] and epiphyseal closure (thereby limiting final height) in both sexes.[11][12]
Reproduction
Female reproductive system
In the female, estradiol acts as a growth hormone for tissue of the reproductive organs, supporting the lining of the vagina, the cervical glands, the endometrium, and the lining of the fallopian tubes. It enhances growth of the myometrium. Estradiol appears necessary to maintain oocytes in the ovary. During the menstrual cycle, estradiol produced by the growing follicle triggers, via a positive feedback system, the hypothalamic-pituitary events that lead to the luteinizing hormone surge, inducing ovulation. In the luteal phase, estradiol, in conjunction with progesterone, prepares the endometrium for implantation. During pregnancy, estradiol increases due to placental production. The effect of estradiol, together with estrone and estriol, in pregnancy is less clear. They may promote uterine blood flow, myometrial growth, stimulate breast growth and at term, promote cervical softening and expression of myometrial oxytocin receptors. In baboons, blocking of estrogen production leads to pregnancy loss, suggesting estradiol has a role in the maintenance of pregnancy. Research is investigating the role of estrogens in the process of initiation of labor. Actions of estradiol are required before the exposure of progesterone in the luteal phase.
Male reproductive system
The effect of estradiol (and estrogens in general) upon male reproduction is complex. Estradiol is produced by action of aromatase mainly in the Leydig cells of the mammalian testis, but also by some germ cells and the Sertoli cells of immature mammals.[14] It functions (in vitro) to prevent apoptosis of male sperm cells.[15] While some studies in the early 1990s claimed a connection between globally declining sperm counts and estrogen exposure in the environment,[16] later studies found no such connection, nor evidence of a general decline in sperm counts.[17][18] Suppression of estradiol production in a subpopulation of subfertile men may improve the semen analysis.[19]
Males with certain sex chromosome genetic conditions, such as Klinefelter's syndrome, will have a higher level of estradiol.[20]
Skeletal system
Estradiol has a profound effect on bone. Individuals without it (or other estrogens) will become tall and eunuchoid, as epiphyseal closure is delayed or may not take place. Bone structure is affected also, resulting in early osteopenia and osteoporosis.[21] Also, women past menopause experience an accelerated loss of bone mass due to a relative estrogen deficiency.[22]
Skin health
The estrogen receptor, as well as the progesterone receptor, have been detected in the skin, including in keratinocytes and fibroblasts.[23][24] At menopause and thereafter, decreased levels of female sex hormones result in atrophy, thinning, and increased wrinkling of the skin and a reduction in skin elasticity, firmness, and strength.[23][24] These skin changes constitute an acceleration in skin aging and are the result of decreased collagen content, irregularities in the morphology of epidermal skin cells, decreased ground substance between skin fibers, and reduced capillaries and blood flow.[23][24] The skin also becomes more dry during menopause, which is due to reduced skin hydration and surface lipids (sebum production).[23] Along with chronological aging and photoaging, estrogen deficiency in menopause is one of the three main factors that predominantly influences skin aging.[23]
HRT, consisting of systemic treatment with estrogen alone or in combination with a progestogen, has well-documented and considerable beneficial effects on the skin of postmenopausal women.[23][24] These benefits include increased skin collagen content, skin thickness and elasticity, and skin hydration and surface lipids.[23][24] Topical estrogen has been found to have similar beneficial effects on the skin.[23] In addition, a study has found that topical 2% progesterone cream significantly increases skin elasticity and firmness and observably decreases wrinkles in peri- and postmenopausal women.[24] Skin hydration and surface lipids, on the other hand, did not significantly change with topical progesterone.[24] These findings suggest that progesterone, like estrogen, also has beneficial effects on the skin, and may be independently protective against skin aging.[24]
Nervous system
Estrogens can be produced in the brain from steroid precursors. As antioxidants, they have been found to have neuroprotective function.[25]
The positive and negative feedback loops of the menstrual cycle involve ovarian estradiol as the link to the hypothalamic-pituitary system to regulate gonadotropins.[26] (See Hypothalamic–pituitary–gonadal axis.)
Estrogen is considered to play a significant role in women’s mental health, with links suggested between the hormone level, mood and well-being. Sudden drops or fluctuations in, or long periods of sustained low levels of estrogen may be correlated with significant mood-lowering. Clinical recovery from depression postpartum, perimenopause, and postmenopause was shown to be effective after levels of estrogen were stabilized and/or restored.[27][28]
Recently, the volumes of sexually dimorphic brain structures in transgender women were found to change and approximate typical female brain structures when exposed to estrogen concomitantly with androgen deprivation over a period of months,[29] suggesting that estrogen and/or androgens have a significant part to play in sex differentiation of the brain, both prenatally and later in life.
There is also evidence the programming of adult male sexual behavior in many vertebrates is largely dependent on estradiol produced during prenatal life and early infancy.[30] It is not yet known whether this process plays a significant role in human sexual behavior, although evidence from other mammals tends to indicate a connection.[31]
Estrogen has been found to increase the secretion of oxytocin and to increase the expression of its receptor, the oxytocin receptor, in the brain.[32] In women, a single dose of estradiol has been found to be sufficient to increase circulating oxytocin concentrations.[33]
Gynecological cancers
Estradiol has been tied to the development and progression of cancers such as breast cancer, ovarian cancer and endometrial cancer. Estradiol affects target tissues mainly by interacting with two nuclear receptors called estrogen receptor α (ERα) and estrogen receptor β (ERβ).[34][35] One of the functions of these estrogen receptors is the modulation of gene expression. Once estradiol binds to the ERs, the receptor complexes then bind to specific DNA sequences, possibly causing damage to the DNA and an increase in cell division and DNA replication. Eukaryotic cells respond to damaged DNA by stimulating or impairing G1, S, or G2 phases of the cell cycle to initiate DNA repair. As a result, cellular transformation and cancer cell proliferation occurs.[36]
Other functions
Estradiol has complex effects on the liver. It affects the production of multiple proteins, including lipoproteins, binding proteins, and proteins responsible for blood clotting. In high amounts, estradiol can lead to cholestasis, for instance cholestasis of pregnancy.
Certain gynecological conditions are dependent on estrogen, such as endometriosis, leiomyomata uteri, and uterine bleeding.
Estrogen affects certain blood vessels. Improvement in arterial blood flow has been demonstrated in coronary arteries.[37]
Biological activity
Estradiol acts primarily as an agonist of the estrogen receptor (ER), a nuclear steroid hormone receptor. There are two subtypes of the ER, ERα and ERβ, and estradiol potently binds to and activates both of these receptors. The result of ER activation is a modulation of gene transcription and expression in ER-expressing cells, which is the predominant mechanism by which estradiol mediates its biological effects in the body. Estradiol also acts as an agonist of membrane estrogen receptors (mERs), such as GPER (GPR30), a recently discovered non-nuclear receptor for estradiol, via which it can mediate a variety of rapid, non-genomic effects.[38] Unlike the case of the ER, GPER appears to be selective for estradiol, and shows very low affinities for other endogenous estrogens, such as estrone and estriol.[39] Additional mERs besides GPER include ER-X, ERx, and Gq-mER.[40][41]
ERα/ERβ are in inactive state trapped in multimolecular chaperone complexes organized around the heat shock protein 90 (HSP90), containing p23 protein, and immunophilin, and located in majority in cytoplasm and partially in nucleus. In the E2 classical pathway or estrogen classical pathway, estradiol enters the cytoplasm, where it interacts with ERs. Once bound E2, ERs dissociate from the molecular chaperone complexes and become competent to dimerize, migrate to nucleus, and to bind to specific DNA sequences (estrogen response element, ERE), allowing for gene transcription which can take place over hours and days.
Estradiol is reported to be approximately 12 times as potent as estrone and 80 times as potent as estriol in its estrogenic activity.[42][43] As such, estradiol is the main estrogen in the body, although the roles of estrone and estriol as estrogens are said to not be negligible.[43]
Biochemistry

Biosynthesis
Estradiol, like other steroids, is derived from cholesterol. After side chain cleavage and using the Δ5 or the Δ4- pathway, Δ4-androstenedione is the key intermediary. A portion of the Δ4-androstenedione is converted to testosterone, which in turn undergoes conversion to estradiol by aromatase. In an alternative pathway, Δ4-androstenedione is aromatized to estrone, which is subsequently converted to estradiol.[45]
During the reproductive years, most estradiol in women is produced by the granulosa cells of the ovaries by the aromatization of Δ4-androstenedione (produced in the theca folliculi cells) to estrone, followed by conversion of estrone to estradiol by 17β-hydroxysteroid dehydrogenase. Smaller amounts of estradiol are also produced by the adrenal cortex, and, in men, by the testes.
Estradiol is not produced in the gonads only, in particular, fat cells produce active precursors to estradiol, and will continue to do so even after menopause.[46] Estradiol is also produced in the brain and in arterial walls.
The biosynthesis of estradiol-like compounds has been observed in leguminous plants, such as Phaseolus vulgaris and soybeans.[47] where they are termed phytoestrogens. Thus, consumption may have oestrogenic effects. In light of this, consumption can be counterproductive to patients undergoing treatment for breast cancer, which usually includes depriving the cancer cells of estrogens.
Distribution
In plasma, estradiol is largely bound to SHBG, and also to albumin. Only a fraction of 2.21% (± 0.04%) is free and biologically active, the percentage remaining constant throughout the menstrual cycle.[48]
Metabolism
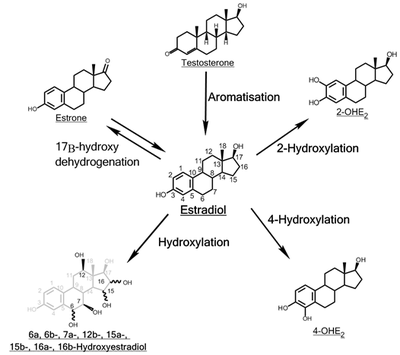
Inactivation of estradiol includes conversion to less-active estrogens, such as estrone and estriol. Estriol is the major urinary metabolite. Estradiol is conjugated in the liver to form estrogen conjugates like estradiol sulfate, estradiol glucuronide and, as such, excreted via the kidneys. Some of the water-soluble conjugates are excreted via the bile duct, and partly reabsorbed after hydrolysis from the intestinal tract. This enterohepatic circulation contributes to maintaining estradiol levels.
Estradiol is also metabolized via hydroxylation into catechol estrogens. In the liver, it is non-specifically metabolized by CYP1A2, CYP3A4, and CYP2C9 via 2-hydroxylation into 2-hydroxyestradiol, and by CYP2C9, CYP2C19, and CYP2C8 via 17β-hydroxy dehydrogenation into estrone,[49] with various other cytochrome P450 (CYP) enzymes and metabolic transformations also being involved.[50]
Estradiol is additionally esterified into lipoidal estradiol forms like estradiol palmitate and estradiol stearate to a certain extent; these esters are stored in adipose tissue and may act as a very long-lasting reservoir of estradiol.[51][52]
Levels
Levels of estradiol in premenopausal women are highly variable throughout the menstrual cycle and reference ranges widely vary from source to source.[53] Estradiol levels are minimal and according to most laboratories range from 20 to 80 pg/mL during the early to mid follicular phase (or the first week of the menstrual cycle, also known as menses).[54][55] Levels of estradiol gradually increase during this time and through the mid to late follicular phase (or the second week of the menstrual cycle) until the pre-ovulatory phase.[53][54] At the time of pre-ovulation (a period of about 24 to 48 hours), estradiol levels briefly surge and reach their highest concentrations of any other time during the menstrual cycle.[53] Circulating levels are typically between 130 and 200 pg/mL at this time, but in some women may be as high as 300 to 400 pg/mL, and the upper limit of the reference range of some laboratories are even greater (for instance, 750 pg/mL).[53][54][56][57][58] Following ovulation (or mid-cycle) and during the latter half of the menstrual cycle or the luteal phase, estradiol levels plateau and fluctuate between around 100 and 150 pg/mL during the early and mid luteal phase, and at the time of the late luteal phase, or a few days before menstruation, reach a low of around 40 pg/mL.[53][55] The mean integrated levels of estradiol during a full menstrual cycle have variously been reported by different sources as 80, 120, and 150 pg/mL.[55][59][60] Although contradictory reports exist, one study found mean integrated estradiol levels of 150 pg/mL in younger women whereas mean integrated levels ranged from 50 to 120 pg/mL in older women.[60]
During the reproductive years of the human female, levels of estradiol are somewhat higher than that of estrone, except during the early follicular phase of the menstrual cycle; thus, estradiol may be considered the predominant estrogen during human female reproductive years in terms of absolute serum levels and estrogenic activity. During pregnancy, estriol becomes the predominant circulating estrogen, and this is the only time at which estetrol occurs in the body, while during menopause, estrone predominates (both based on serum levels). The estradiol produced by male humans, from testosterone, is present at serum levels roughly comparable to those of postmenopausal women (14-55 versus <35 pg/mL, respectively). It has also been reported that if concentrations of estradiol in a 70-year-old man are compared to those of a 70-year-old woman, levels are approximately 2- to 4-fold higher in the man.[61]
Measurement
In women, serum estradiol is measured in a clinical laboratory and reflects primarily the activity of the ovaries. As such, they are useful in the detection of baseline estrogen in women with amenorrhea or menstrual dysfunction, and to detect the state of hypoestrogenicity and menopause. Furthermore, estrogen monitoring during fertility therapy assesses follicular growth and is useful in monitoring the treatment. Estrogen-producing tumors will demonstrate persistent high levels of estradiol and other estrogens. In precocious puberty, estradiol levels are inappropriately increased.
Ranges
Individual laboratory results should always been interpreted using the ranges provided by the laboratory that performed the test.

- The ranges denoted By biological stage may be used in closely monitored menstrual cycles in regard to other markers of its biological progression, with the time scale being compressed or stretched to how much faster or slower, respectively, the cycle progresses compared to an average cycle.
- The ranges denoted Inter-cycle variability are more appropriate to use in unmonitored cycles with only the beginning of menstruation known, but where the woman accurately knows her average cycle lengths and time of ovulation, and that they are somewhat averagely regular, with the time scale being compressed or stretched to how much a woman's average cycle length is shorter or longer, respectively, than the average of the population.
- The ranges denoted Inter-woman variability are more appropriate to use when the average cycle lengths and time of ovulation are unknown, but only the beginning of menstruation is given.[62]
| Reference ranges for serum estradiol | |||
|---|---|---|---|
| Patient type | Lower limit | Upper limit | Unit |
| Adult male | 50[63] | 200[63] | pmol/L |
| 14 | 55 | pg/mL | |
| Adult female (follicular phase, day 5) | 70[63] 95% PI (standard) | 500[63] 95% PI | pmol/L |
| 110[64] 90% PI (used in diagram) | 220[64] 90% PI | ||
| 19 (95% PI) | 140 (95% PI) | pg/mL | |
| 30 (90% PI) | 60 (90% PI) | ||
| Adult female (preovulatory peak) | 400[63] | 1500[63] | pmol/L |
| 110 | 410 | pg/mL | |
| Adult female (luteal phase) | 70[63] | 600[63] | pmol/L |
| 19 | 160 | pg/mL | |
| Adult female - free (not protein bound) | 0.5[65] | 9[65] | pg/mL |
| 1.7[65] | 33[65] | pmol/L | |
| Post-menopausal female | N/A[63] | < 130[63] | pmol/L |
| N/A | < 35 | pg/mL | |
In the normal menstrual cycle, estradiol levels measure typically <50 pg/ml at menstruation, rise with follicular development (peak: 200 pg/ml), drop briefly at ovulation, and rise again during the luteal phase for a second peak. At the end of the luteal phase, estradiol levels drop to their menstrual levels unless there is a pregnancy.
During pregnancy, estrogen levels, including estradiol, rise steadily toward term. The source of these estrogens is the placenta, which aromatizes prohormones produced in the fetal adrenal gland.
Medical use
Estradiol is used as a medication, mainly in hormone replacement therapy.[66]
Chemistry
Estradiol is an estrane (C18) steroid.[66] It is also known as 17β-estradiol (to distinguish it from 17α-estradiol) or as estra-1,3,5(10)-triene-3,17β-diol. It has two hydroxyl groups, one at the C3 position and the other at the 17β position, as well as three double bonds in the A ring. Due to its two hydroxyl groups, estradiol is often abbreviated as E2. The structurally related estrogens, estrone (E1), estriol (E3), and estetrol (E4) have one, three, and four hydroxyl groups, respectively.
History
The discovery of estrogen is usually credited to the American scientists Edgar Allen and Edward A. Doisy.[67][68] In 1923, they observed that injection of fluid from porcine ovarian follicles produced pubertal- and estrus-type changes (including vaginal, uterine, and mammary gland changes and sexual receptivity) in sexually immature, ovariectomized mice and rats.[67][68][69] These findings demonstrated the existence of a hormone which is produced by the ovaries and is involved in sexual maturation and reproduction.[67][68][69] At the time of its discovery, Allen and Doisy did not name the hormone, and simply referred to it as an "ovarian hormone" or "follicular hormone";[68] others referred to it variously as feminin, folliculin, menformon, thelykinin, and emmenin.[70][71] In 1926, Parkes and Bellerby coined the term estrin to describe the hormone on the basis of it inducing estrus in animals.[72][70] Estrone was isolated and purified independently by Allen and Doisy and German scientist Adolf Butenandt in 1929, and estriol was isolated and purified by Marrian in 1930; they were the first estrogens to be identified.[68][73][74]
Estradiol, the most potent of the three major estrogens, was the last of the three to be identified.[68][72] It was discovered by Schwenk and Hildebrant in 1933, who synthesized it via reduction of estrone.[68] Estradiol was subsequently isolated and purified from sow ovaries by Doisy in 1935, with its chemical structure determined simultaneously,[75] and was referred to variously as dihydrotheelin, dihydrofolliculin, and dihydroxyestrin.[68][76] In 1935, the name estradiol and the term estrogen were formally established by the Sex Hormone Committee of the Health Organization of the League of Nations; this followed the names estrone (which was initially called theelin, progynon, folliculin, and ketohydroxyestrin) and estriol (initially called theelol and trihydroxyestrin) having been established in 1932 at the first meeting of the International Conference on the Standardization of Sex Hormones in London.[72][77] Following its discovery, a partial synthesis of estradiol from cholesterol was developed by Inhoffen and Hohlweg in 1940, and a total synthesis was developed by Anner and Miescher in 1948.[68]
In 1931, Butenandt found that the benzoic acid ester of estrone had a prolonged duration of action.[78][79] Subsequently, Schwenk and Hildebrant synthesized estradiol benzoate from estradiol in 1933,[80][81] and estradiol benzoate was introduced by Schering-Kahlbaum for medical use via intramuscular injection under the brand name Progynon-B in 1936.[82] It was the first estrogen ester to be marketed,[83] and has since been followed by many additional esters, for instance estradiol valerate and estradiol cypionate in the 1950s.[84][85][86] Ethinylestradiol was synthesized from estradiol by Inhoffen and Hohlweg in 1938 and was introduced for oral use by Schering in the United States under the brand name Estinyl in 1943.[80][87] It remains widely used in combined oral contraceptives.[80]
Society and culture
Etymology
The name estradiol derives from estra-, Gk. οἶστρος (oistros, literally meaning "verve or inspiration"),[88] which refers to the estrane steroid ring system, and -diol, a chemical term and suffix indicating that the compound is a type of alcohol bearing two hydroxyl groups.
References
- ↑ Susan M. Ford; Sally S. Roach (7 October 2013). Roach's Introductory Clinical Pharmacology. Lippincott Williams & Wilkins. pp. 525–. ISBN 978-1-4698-3214-2.
- ↑ Maryanne Hochadel; Mosby (1 April 2015). Mosby's Drug Reference for Health Professions. Elsevier Health Sciences. pp. 602–. ISBN 978-0-323-31103-8.
- 1 2 3 4 5 Stanczyk, Frank Z.; Archer, David F.; Bhavnani, Bhagu R. (2013). "Ethinyl estradiol and 17β-estradiol in combined oral contraceptives: pharmacokinetics, pharmacodynamics and risk assessment". Contraception. 87 (6): 706–727. ISSN 0010-7824. PMID 23375353. doi:10.1016/j.contraception.2012.12.011.
- ↑ Tommaso Falcone; William W. Hurd (2007). Clinical Reproductive Medicine and Surgery. Elsevier Health Sciences. pp. 22–. ISBN 0-323-03309-1.
- ↑ Price, T; Blauer, K; Hansen, M; Stanczyk, F; Lobo, R; Bates, G (1997). "Single-dose pharmacokinetics of sublingual versus oral administration of micronized 17-estradiol". Obstetrics & Gynecology. 89 (3): 340–345. ISSN 0029-7844. PMID 9052581. doi:10.1016/S0029-7844(96)00513-3.
- ↑ Naunton, Mark; Al Hadithy, Asmar F. Y.; Brouwers, Jacobus R. B. J.; Archer, David F. (2006). "Estradiol gel". Menopause. 13 (3): 517–527. ISSN 1072-3714. doi:10.1097/01.gme.0000191881.52175.8c.
- ↑ Ryan KJ (August 1982). "Biochemistry of aromatase: significance to female reproductive physiology". Cancer Res. 42 (8 Suppl): 3342s–3344s. PMID 7083198.
- ↑ Mechoulam R, Brueggemeier RW, Denlinger DL (September 1984). "Estrogens in insects" (PDF). Cellular and Molecular Life Sciences. 40 (9): 942–944. doi:10.1007/BF01946450.
- ↑ Ozon R (1972). "Estrogens in Fishes, Amphibians, Reptiles, and Birds". In Idler DR. Steroids In Nonmammalian Vertebrates. Oxford: Elsevier Science. pp. 390–414. ISBN 032314098X.
- ↑ Saldanha, Colin J., Luke Remage-Healey, and Barney A. Schlinger. "Synaptocrine signaling: steroid synthesis and action at the synapse." Endocrine reviews 32.4 (2011): 532-549.
- 1 2 3 Julia A. McMillan; Ralph D. Feigin; Catherine DeAngelis; M. Douglas Jones (2006). Oski's Pediatrics: Principles & Practice. Lippincott Williams & Wilkins. pp. 550–. ISBN 978-0-7817-3894-1.
- 1 2 3 Charles R. Craig; Robert E. Stitzel (2004). Modern Pharmacology with Clinical Applications. Lippincott Williams & Wilkins. pp. 706–. ISBN 978-0-7817-3762-3.
- ↑ Victor R. Preedy (2 December 2011). Handbook of Growth and Growth Monitoring in Health and Disease. Springer Science & Business Media. pp. 2661–. ISBN 978-1-4419-1794-2.
- ↑ Carreau S, Lambard S, Delalande C, Denis-Galeraud I, Bilinska B, Bourguiba S (2003). "Aromatase expression and role of estrogens in male gonad : a review". Reproductive Biology and Endocrinology. 1: 35. PMC 155680
 . PMID 12747806. doi:10.1186/1477-7827-1-35.
. PMID 12747806. doi:10.1186/1477-7827-1-35. - ↑ Pentikäinen V, Erkkilä K, Suomalainen L, Parvinen M, Dunkel L (2000). "Estradiol acts as a germ cell survival factor in the human testis in vitro". The Journal of Clinical Endocrinology and Metabolism. 85 (5): 2057–67. PMID 10843196. doi:10.1210/jcem.85.5.6600.
- ↑ Sharpe RM, Skakkebaek NE (1993). "Are oestrogens involved in falling sperm counts and disorders of the male reproductive tract?". Lancet. 341 (8857): 1392–5. PMID 8098802. doi:10.1016/0140-6736(93)90953-E.
- ↑ Handelsman, DJ (2001). "Estrogens and falling sperm counts.". Reproduction, Fertility and Development. 13 (4): 317–24. PMID 11800170.
- ↑ Fisch, Harry; Goldstein, Robert (2003). "Environmental estrogens and sperm counts" (PDF). Pure Applied Chemistry. 75 (11–12): 2181–2193. doi:10.1351/pac200375112181.
- ↑ Raman JD, Schlegel PN (2002). "Aromatase inhibitors for male infertility". The Journal of Urology. 167 (2 Pt 1): 624–9. PMID 11792932. doi:10.1016/S0022-5347(01)69099-2.
- ↑ Visootsak J, Graham JM (2006). "Klinefelter syndrome and other sex chromosomal anueploidies". Orphanet Journal of Rare Diseases. 1 (42): 42. PMC 1634840
 . PMID 17062147. doi:10.1186/1750-1172-1-42. Retrieved 20 November 2013.
. PMID 17062147. doi:10.1186/1750-1172-1-42. Retrieved 20 November 2013. - ↑ Carani C, Qin K, Simoni M, Faustini-Fustini M, Serpente S, Boyd J, Korach KS, Simpson ER (1997). "Effect of testosterone and estradiol in a man with aromatase deficiency". The New England Journal of Medicine. 337 (2): 91–5. PMID 9211678. doi:10.1056/NEJM199707103370204.
- ↑ Albright, Fuller; Smith Patricia H.; Richardson Anna M. (31 May 1941). "Postmenopausal Osteoporosis: Its Clinical Features". JAMA. 116 (22): 2465–2474. doi:10.1001/jama.1941.02820220007002. Retrieved 20 November 2013.
- 1 2 3 4 5 6 7 8 Raine-Fenning NJ, Brincat MP, Muscat-Baron Y (2003). "Skin aging and menopause : implications for treatment". Am J Clin Dermatol. 4 (6): 371–8. PMID 12762829. doi:10.2165/00128071-200304060-00001.
- 1 2 3 4 5 6 7 8 Holzer G, Riegler E, Hönigsmann H, Farokhnia S, Schmidt JB, Schmidt B (2005). "Effects and side-effects of 2% progesterone cream on the skin of peri- and postmenopausal women: results from a double-blind, vehicle-controlled, randomized study". Br. J. Dermatol. 153 (3): 626–34. PMID 16120154. doi:10.1111/j.1365-2133.2005.06685.x.
- ↑ Behl C, Widmann M, Trapp T, Holsboer F (November 1995). "17-beta estradiol protects neurons from oxidative stress-induced cell death in vitro". Biochem. Biophys. Res. Commun. 216 (2): 473–82. PMID 7488136. doi:10.1006/bbrc.1995.2647.
- ↑ Meethal, S. V.; Liu, T.; Chan, H. W.; Ginsburg, E.; Wilson, A. C.; Gray, D. N.; Bowen, R. L.; Vonderhaar, B. K.; Atwood, C. S. (2009). "Identification of a regulatory loop for the synthesis of neurosteroids: A steroidogenic acute regulatory protein-dependent mechanism involving hypothalamic-pituitary-gonadal axis receptors". Journal of Neurochemistry. 110 (3): 1014–1027. PMC 2789665
 . PMID 19493163. doi:10.1111/j.1471-4159.2009.06192.x.
. PMID 19493163. doi:10.1111/j.1471-4159.2009.06192.x. - ↑ Douma SL, Husband C, O'Donnell ME, Barwin BN, Woodend AK (2005). "Estrogen-related mood disorders: reproductive life cycle factors". Adv Nurs Sci. 28 (4): 364–75. PMID 16292022. doi:10.1097/00012272-200510000-00008.
- ↑ Lasiuk GC, Hegadoren KM (October 2007). "The effects of estradiol on central serotonergic systems and its relationship to mood in women". Biol Res Nurs. 9 (2): 147–60. PMID 17909167. doi:10.1177/1099800407305600.
- ↑ Hulshoff HE, Cohen-Kettenis PT, Van Haren NE, Peper JS, Brans RG, Cahn W, Schnack HG, Gooren LJ, Kahn RS (July 2006). "Changing your sex changes your brain: influences of testosterone and estrogen on adult human brain structure". European Journal of Endocrinology. 155 (suppl_1): 107–114. doi:10.1530/eje.1.02248.
- ↑ Harding CF (June 2004). "Hormonal Modulation of Singing: Hormonal Modulation of the Songbird Brain and Singing Behavior". Ann. N.Y. Acad. Sci. The New York Academy of Sciences. 1016: 524–539. PMID 15313793. doi:10.1196/annals.1298.030. Retrieved 2007-03-07.
- ↑ Simerly RB (2002-03-27). "Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain" (pdf). Annu. Rev. Neurosci. 25: 507–536. PMID 12052919. doi:10.1146/annurev.neuro.25.112701.142745. Retrieved 2007-03-07.
- ↑ Goldstein I, Meston CM, Davis S, Traish A (17 November 2005). Women's Sexual Function and Dysfunction: Study, Diagnosis and Treatment. CRC Press. pp. 205–. ISBN 978-1-84214-263-9.
- ↑ Acevedo-Rodriguez A, Mani SK, Handa RJ (2015). "Oxytocin and Estrogen Receptor β in the Brain: An Overview". Frontiers in Endocrinology. 6: 160. PMC 4606117
 . PMID 26528239. doi:10.3389/fendo.2015.00160.
. PMID 26528239. doi:10.3389/fendo.2015.00160. - ↑ Bulzomi P, Bolli A, Galluzzo P, Leone S, Acconcia F, Marino M (January 2010). "Naringenin and 17β-estradiol coadministration prevents hormone-induced human cancer cell growth". IUBMD Life. 62 (1): 51–60. PMID 19960539. doi:10.1002/iub.279.
- ↑ Sreeja S, Santhosh Kumar TR, Lakshmi BS, Sreeja S (17 March 2011). "Pomegranate extract demonstrate a selective estrogen receptor modulator profile in human tumor cell lines and in vivo models of estrogen deprivation". Journal of Nutritional Biochemistry. 23 (7): 725–32. PMID 21839626. doi:10.1016/j.jnutbio.2011.03.015.
- ↑ Thomas CG, Strom A, Lindberg K, Gustafsson JA (22 June 2010). "Estrogen receptor beta decreases survival of p53-defective cancer cells after DNA damage by impairing G2/M checkpoint signaling". Breast Cancer Research and Treatment. 127 (2): 417–427. PMID 20623183. doi:10.1007/s10549-010-1011-z.
- ↑ Collins P, Rosano GM, Sarrel PM, Ulrich L, Adamopoulos S, Beale CM, McNeill JG, Poole-Wilson PA (1995). "17 beta-Estradiol attenuates acetylcholine-induced coronary arterial constriction in women but not men with coronary heart disease". Circulation. 92 (1): 24–30. PMID 7788912. doi:10.1161/01.CIR.92.1.24.
- ↑ Prossnitz ER, Barton M (May 2014). "Estrogen biology: New insights into GPER function and clinical opportunities". Mol. Cell. Endocrinol. 389 (1–2): 71–83. PMC 4040308
 . PMID 24530924. doi:10.1016/j.mce.2014.02.002.
. PMID 24530924. doi:10.1016/j.mce.2014.02.002. - ↑ Prossnitz ER, Arterburn JB, Sklar LA (2007). "GPR30: A G protein-coupled receptor for estrogen". Mol. Cell. Endocrinol. 265-266: 138–42. PMC 1847610
 . PMID 17222505. doi:10.1016/j.mce.2006.12.010.
. PMID 17222505. doi:10.1016/j.mce.2006.12.010. - ↑ Soltysik K, Czekaj P (April 2013). "Membrane estrogen receptors - is it an alternative way of estrogen action?". J. Physiol. Pharmacol. 64 (2): 129–42. PMID 23756388.
- ↑ Micevych PE, Kelly MJ (2012). "Membrane estrogen receptor regulation of hypothalamic function". Neuroendocrinology. 96 (2): 103–10. PMC 3496782
 . PMID 22538318. doi:10.1159/000338400.
. PMID 22538318. doi:10.1159/000338400. - ↑ Susan Tucker Blackburn (2007). Maternal, Fetal, & Neonatal Physiology: A Clinical Perspective. Elsevier Health Sciences. pp. 43–. ISBN 1-4160-2944-3.
- 1 2 John E. Hall (31 May 2015). Guyton and Hall Textbook of Medical Physiology E-Book. Elsevier Health Sciences. pp. 1043–. ISBN 978-0-323-38930-3.
- ↑ Häggström, Mikael; Richfield, David (2014). "Diagram of the pathways of human steroidogenesis". WikiJournal of Medicine. 1 (1). ISSN 2002-4436. doi:10.15347/wjm/2014.005.
- ↑ Walter F. Boron; Emile L. Boulpaep (2003). Medical Physiology: A Cellular And Molecular Approach. Elsevier/Saunders. p. 1300. ISBN 1-4160-2328-3.
- ↑ Mutschler, Ernst; Schäfer-Korting, Monika (2001). Arzneimittelwirkungen (in German) (8 ed.). Stuttgart: Wissenschaftliche Verlagsgesellschaft. pp. 434, 444. ISBN 3-8047-1763-2.
- ↑ Young, I. J.; Hillman, J. R.; Knights, B. A. (1978). "Endogenous Estradiol-17 β in Phaseolus vulgaris". Zeitschrift für Pflanzenphysiologie. 90: 45–50. doi:10.1016/S0044-328X(78)80223-2.
- ↑ Wu CH, Motohashi T, Abdel-Rahman HA, Flickinger GL, Mikhail G (August 1976). "Free and protein-bound plasma estradiol-17 beta during the menstrual cycle". J. Clin. Endocrinol. Metab. 43 (2): 436–45. PMID 950372. doi:10.1210/jcem-43-2-436.
- ↑ Cheng ZN, Shu Y, Liu ZQ, Wang LS, Ou-Yang DS, Zhou HH (February 2001). "Role of cytochrome P450 in estradiol metabolism in vitro". Acta Pharmacol. Sin. 22 (2): 148–54. PMID 11741520.
- ↑ Lee AJ, Cai MX, Thomas PE, Conney AH, Zhu BT (August 2003). "Characterization of the oxidative metabolites of 17beta-estradiol and estrone formed by 15 selectively expressed human cytochrome p450 isoforms". Endocrinology. 144 (8): 3382–98. PMID 12865317. doi:10.1210/en.2003-0192.
- ↑ Michael Oettel; Ekkehard Schillinger (6 December 2012). Estrogens and Antiestrogens I: Physiology and Mechanisms of Action of Estrogens and Antiestrogens. Springer Science & Business Media. pp. 235–237. ISBN 978-3-642-58616-3.
- ↑ Michael Oettel; Ekkehard Schillinger (6 December 2012). Estrogens and Antiestrogens II: Pharmacology and Clinical Application of Estrogens and Antiestrogen. Springer Science & Business Media. pp. 268, 271. ISBN 978-3-642-60107-1.
- 1 2 3 4 5 Jill B. Becker; Karen J. Berkley; Nori Geary; Elizabeth Hampson; James P. Herman; Elizabeth Young (4 December 2007). Sex Differences in the Brain: From Genes to Behavior. Oxford University Press. pp. 64–. ISBN 978-0-19-804255-6.
Estradiol levels are minimal during the earliest days of the follicular phase, but increasing concentrations are released into the general circulation as the follicle matures. The highest levels are reached about 24 to 48 hours before the LH peak. In fact, the pre-ovulatory peak in estradiol represents its highest concentration during the entire menstrual cycle. Serum concentrations at this time are typically about 130-200 pg/mL, but concentrations as high as 300-400 pg/mL can be achieved in some women. Following a transient fall in association with ovulation, estradiol secretion is restored by production from the corpus luteum during the luteal phase. Plateau levels of around 100-150 pg/mL (Abraham, 1978; Thorneycroft et al., 1971) are most often seen during the period from -10 to -5 days before the onset of menses. With the regression of the corpus luteum, estradiol levels fall, gradually in some women and precipitously in others, during the last few days of the luteal phase. This ushers in the onset of menses, the sloughing of the endometrium. Serum estradiol during menses is approximately 30-50 pg/mL. (Source.)
- 1 2 3 Jerome Frank Strauss; Robert L. Barbieri (2009). Yen and Jaffe's Reproductive Endocrinology: Physiology, Pathophysiology, and Clinical Management. Elsevier Health Sciences. pp. 807–. ISBN 1-4160-4907-X.
In most laboratories, serum estradiol levels range from 20 to 80 pg/mL during the early to midfollicular phase of the menstrual cycle and peak at 200 to 500 pg/mL during the preovulatory surge. During the midluteal phase, serum estradiol levels range from 60 to 200 pg/mL.
- 1 2 3 C. Christian; B. von Schoultz (15 March 1994). Hormone Replacement Therapy: Standardized or Individually Adapted Doses?. CRC Press. pp. 60–. ISBN 978-1-85070-545-1.
Plasma levels of estradiol range from 40 to 80 pg/ml during the 1st week of the ovarian cycle (early follicular phase) and from 80 to 300 pg/ml during the 2nd week (mid- and late follicular phase including periovulatory peak). Then during the 3rd and 4th weeks, estradiol fluctuates between 100 and 150 pg/ml (early and mid-luteal phase) to 40 pg/ml a few days before menstruation (late luteal phase). The mean integrated estradiol level during a full 28-day normal cycle is around 80 pg/ml.
- ↑ J. Larry Jameson; Leslie J. De Groot (18 May 2010). Endocrinology: Adult and Pediatric. Elsevier Health Sciences. pp. 2812–. ISBN 1-4557-1126-8.
Midcycle: 150-750 pg/mL
- ↑ Ian D. Hay; John A. H. Wass (26 January 2009). Clinical Endocrine Oncology. John Wiley & Sons. pp. 623–. ISBN 978-1-4443-0023-9.
Mid-cycle: 110-330 pg/mL
- ↑ Robert F. Dons (12 July 1994). Endocrine and Metabolic Testing Manual. CRC Press. pp. 8–. ISBN 978-0-8493-7657-3.
Ovulatory: 200-400 pg/mL
- ↑ M. Notelovitz; P.A. van Keep (6 December 2012). The Climacteric in Perspective: Proceedings of the Fourth International Congress on the Menopause, held at Lake Buena Vista, Florida, October 28–November 2, 1984. Springer Science & Business Media. pp. 397–. ISBN 978-94-009-4145-8.
[...] following the menopause, circulating estradiol levels decrease from a premenopausal mean of 120 pg/ml to only 13 pg/ml.
- 1 2 Eugenio E. Müller; Robert M. MacLeod (6 December 2012). Neuroendocrine Perspectives. Springer Science & Business Media. pp. 121–. ISBN 978-1-4612-3554-5.
[...] [premenopausal] mean [estradiol] concentration of 150 pg/ml [...]
- ↑ Sayed Y, Taxel P (2003). "The use of estrogen therapy in men". Curr Opin Pharmacol. 3 (6): 650–4. PMID 14644018.
- ↑ Häggström, Mikael (2014). "Reference ranges for estradiol, progesterone, luteinizing hormone and follicle-stimulating hormone during the menstrual cycle". WikiJournal of Medicine. 1 (1). ISSN 2002-4436. doi:10.15347/wjm/2014.001.
- 1 2 3 4 5 6 7 8 9 10 GPNotebook — reference range (oestradiol) Retrieved on September 27, 2009
- 1 2 Values taken from day 1 after LH surge in: Stricker R, Eberhart R, Chevailler MC, Quinn FA, Bischof P, Stricker R (2006). "Establishment of detailed reference values for luteinizing hormone, follicle stimulating hormone, estradiol, and progesterone during different phases of the menstrual cycle on the Abbott ARCHITECT analyzer". Clin. Chem. Lab. Med. 44 (7): 883–7. PMID 16776638. doi:10.1515/CCLM.2006.160. as PDF
- 1 2 3 4 Total amount multiplied by 0.022 according to 2.2% presented in: Wu CH, Motohashi T, Abdel-Rahman HA, Flickinger GL, Mikhail G (August 1976). "Free and protein-bound plasma estradiol-17 beta during the menstrual cycle". J. Clin. Endocrinol. Metab. 43 (2): 436–45. PMID 950372. doi:10.1210/jcem-43-2-436.
- 1 2 Kuhl H (2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration". Climacteric. 8 Suppl 1: 3–63. PMID 16112947. doi:10.1080/13697130500148875.
- 1 2 3 D. Lynn Loriaux; Lynn Loriaux (14 March 2016). A Biographical History of Endocrinology. John Wiley & Sons. pp. 345–. ISBN 978-1-119-20246-2.
- 1 2 3 4 5 6 7 8 9 Christian Lauritzen; John W. W. Studd (22 June 2005). Current Management of the Menopause. CRC Press. pp. 44–. ISBN 978-0-203-48612-2.
- 1 2 Allen, Edgar; Doisy, Edward A. (1923). "AN OVARIAN HORMONE". Journal of the American Medical Association. 81 (10): 819. ISSN 0002-9955. doi:10.1001/jama.1923.02650100027012.
- 1 2 J.G. Gruhn; R.R. Kazer (11 November 2013). Hormonal Regulation of the Menstrual Cycle: The Evolution of Concepts. Springer Science & Business Media. pp. 69–73. ISBN 978-1-4899-3496-3.
- ↑ Newerla, Gerhard J. (1944). "The History of the Discovery and Isolation of the Female Sex Hormones". New England Journal of Medicine. 230 (20): 595–604. ISSN 0028-4793. doi:10.1056/NEJM194405182302001.
- 1 2 3 Marc A. Fritz; Leon Speroff (28 March 2012). Clinical Gynecologic Endocrinology and Infertility. Lippincott Williams & Wilkins. pp. 750–. ISBN 978-1-4511-4847-3.
- ↑ Fritz F. Parl (2000). Estrogens, Estrogen Receptor and Breast Cancer. IOS Press. pp. 4–. ISBN 978-0-9673355-4-4.
- ↑ Alan C. Sartorelli; David G. Johns (27 November 2013). Antineoplastic and Immunosuppressive Agents. Springer Science & Business Media. pp. 104–. ISBN 978-3-642-65806-8.
- ↑ Donna Shoupe; Florence P. Haseltine (6 December 2012). Contraception. Springer Science & Business Media. pp. 2–. ISBN 978-1-4612-2730-4.
- ↑ MacCorquodale, D. W.; Thayer, S. A.; Doisy, E. A. (1935). "The Crystalline Ovarian Follicular Hormone". Experimental Biology and Medicine. 32 (7): 1182–1182. ISSN 1535-3702. doi:10.3181/00379727-32-8020P.
- ↑ Anne Fausto-Sterling (2000). Sexing the Body: Gender Politics and the Construction of Sexuality. Basic Books. pp. 189–. ISBN 978-0-465-07714-4.
- ↑ A. Labhart (6 December 2012). Clinical Endocrinology: Theory and Practice. Springer Science & Business Media. pp. 512–. ISBN 978-3-642-96158-8.
- ↑ Butenandt, A.; Hildebrandt, F. (1931). "Über ein zweites Hormonkrystallisat aus Schwangerenharn und seine physiologischen und chemischen Beziehungen zum krystallisierten Follikelhormon. [Untersuchungen über das weibliche Sexualhormon, 6. Mitteilung.]". Hoppe-Seyler´s Zeitschrift für physiologische Chemie. 199 (4-6): 243–265. ISSN 0018-4888. doi:10.1515/bchm2.1931.199.4-6.243.
- 1 2 3 Michael Oettel; Ekkehard Schillinger (6 December 2012). Estrogens and Antiestrogens I: Physiology and Mechanisms of Action of Estrogens and Antiestrogens. Springer Science & Business Media. pp. 7–8. ISBN 978-3-642-58616-3.
- ↑ Miescher K, Scholz C, Tschopp E (1938). "The activation of female sex hormones: Mono-esters of alpha-oestradiol". Biochem. J. 32 (8): 1273–80. PMC 1264184
 . PMID 16746750.
. PMID 16746750. - ↑ Enrique Raviña; Hugo Kubinyi (16 May 2011). The Evolution of Drug Discovery: From Traditional Medicines to Modern Drugs. John Wiley & Sons. p. 175. ISBN 978-3-527-32669-3. Retrieved 20 May 2012.
- ↑ Walter Sneader (23 June 2005). Drug Discovery: A History. John Wiley & Sons. pp. 195–. ISBN 978-0-471-89979-2.
- ↑ Index Nominum 2000: International Drug Directory. Taylor & Francis US. 2000. p. 404. ISBN 978-3-88763-075-1. Retrieved 29 May 2012.
- ↑ J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 897–. ISBN 978-1-4757-2085-3.
- ↑ Oriowo MA, Landgren BM, Stenström B, Diczfalusy E (1980). "A comparison of the pharmacokinetic properties of three estradiol esters". Contraception. 21 (4): 415–24. PMID 7389356.
- ↑ Mosby's GenRx: A Comprehensive Reference for Generic and Brand Prescription Drugs. Mosby. 2001. p. 944. ISBN 978-0-323-00629-3.
- ↑ "Greek Word Study Tool: oistros". Perseus Digital Library. Retrieved 2011-12-28.
External links
- Estradiol MS Spectrum
- Estrogens - Lab Tests Online