Erythrogenic toxin
Erythrogenic toxins, also referred to as streptococcal pyrogenic exotoxins, are secreted by strains of the bacterium Streptococcus pyogenes.[1][2] SpeA and SpeC are superantigens, which induce inflammation by nonspecifically activating T cells and stimulating the production of inflammatory cytokines.[3] SpeB, the most abundant streptococcal extracellular protein, is a cysteine protease.[4][5] Pyrogenic exotoxins are implicated as the causative agent of scarlet fever and streptococcal toxic shock syndrome.[2] There is no consensus on the exact number of pyrogenic exotoxins. Serotypes A-C are the most extensively studied and recognized by all sources, but others note up to thirteen distinct types, categorizing SpeF-M as additional superantigens.[1][2][6][7]
Erythrogenic toxins are known to damage the plasma membranes of blood capillaries under the skin and produce a red skin rash (characteristic of scarlet fever).[8] Past studies have shown that multiple variants of erythrogenic toxins may be produced, depending on the strain of S. pyogenes in question. Some strains may not produce a detectable toxin at all.[9] Bacteriophage T12 infection of Streptococcus pyogenes enables the production of Spe A, and increases virulence.[10]
History
Discovery and Nomenclature
SpeB was identified in 1919 as an ectoenzyme secreted by certain strains of streptococci.[11] It was originally studied as two separate toxins, streptococcal pyrogenic exotoxin B and streptococcal cysteine proteinase, until it was shown that both proteins were encoded by the speB gene and that the attributed pyrogenic activities were due to contamination by SpeA and SpeC.[12]
Pyrogenic, in streptococcal pyrogenic exotoxin, means "causes fever."[13] Erythrogenic refers to the typical red rash of scarlet fever. In older literature, these toxins are also referred to as scarlatina toxins or scarlet fever toxins due to their role as the causative agents of the disease.[2]
SpeB is known as streptococcal pyrogenic exotoxin B, streptopain and streptococcal cysteine proteinase as a result of its original misidentification as two separate toxins, and is neither an exotoxin nor pyrogenic.[12]
Structure
Location of Genes
The speB and speJ genes are located in the core bacterial chromosome of all strains of S. pyogenes.[3][14] However, despite its presence and high levels of conservation in the nucleotide sequence, 25-40% of these strains do not express the SpeB toxin in significant amounts.[14]
In contrast, speA, speC and speH-M are encoded by bacteriophages.[3][15]
There is a lack of consensus over the location of the speG gene, which has been attributed to both the core chromosome and lysogenic phages.[1]
Protein Structure
SpeB is a 28 kDa protein with three major forms, mSpeB1, mSpeB2 and mSpeB3, which are categorized by variations the primary amino acid sequence.[4] Three amino acids, C192, H340, and W357, are vital for enzymatic activity in all variants.[11] The toxin contains a canonical papain-like domain, and mSpeB2 has an additional human integrin binding domain.[4][11]

All superantigenic streptococcal pyrogenic exotoxins contain two major conserved protein domains that are linked by an α-helix, which consist of an amino-terminal oligosoccharide/oligonucleotide binding fold and a carboxy-terminal β-grasp domain, as well as dodecapeptide binding region. SpeA also has a cystine loop, a low-affinity α-chain MHC II binding site, and the Vβ-TCR binding site. SpeC, SpeG, SpeH and SpeJ contains a Zn2+-dependent high β-chain MHC II binding site in addition to the low affinity site present in SpeA, and lacks the cystine loop. SpeH also has an additional α3-β8 loop that mediates the specificity of the toxin's Vβ-TCR binding site.[2]
Processing and Regulation
The speB gene encodes for an amino acid sequence that becomes the 40 kDa zymogen, known as SpeBz, after cleavage of the signal sequence.[11] SpeBz undergoes autocatalysis through at least eight intermediates to create the 28 kDa SpeBm. Finally cystine-192 and histidine-340 form a catalytic dyad.[4][5] Each step is tightly regulated by multiple factors, allowing sophisticated temporal expression of the mature proteinase.[11]
Mechanisms of Action

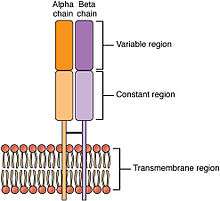
SpeA and SpeC
SpeA and SpeC bind to MHC Class II molecules, are presented to T cells, and bind to the variable region of the beta chain of T cell receptors.[3] Once activated, the T cells release pro-inflammatory cytokines and chemokines.[1] The interactions with TCRs are characterized by low affinities and fast dissociation, allowing the toxin to activate multiple T cells in succession.[16] The lack of specificity allow the activation of up to 50% of the T cells in the body.[6]
SpeB
SpeB cleaves degrades multiple proteins through hydrolysis, including cytokines, extracellular matrix proteins and immunoglobulin.[12] It requires three amino acids before the cleavage site, known as P1, P2 and P3. Of these, SpeB has a preference for hydrophobic P2 and positively charged P1 residues, with greater importance of the P2 amino acid.[5][11]
Roles in Virulence, Pathogenesis and Infection
SpeB
Streptococcal cysteine proteinase has roles in immune evasion and apoptosis, as well as potential influence on bacterial internalization. There is contradictory evidence regarding the effect of SpeB on virulence. Some studies have reported increased protease levels in strains that cause scarlet fever in comparison to those associated with streptococcal toxic shock syndrome, while others show decreased expression in more virulent strains.[4]
SpeB degrades immunoglobulins and cytokines, as well as through cleavage of C3b, inhibiting recruitment of phagocytic cells and the complement activation pathway.[5] This results in decreased inflammation and neutrophil levels around the site of infection, preventing clearance and through phagocytosis and promoting the survival of S. pyogenes.[4][5]
The toxin also induces apoptosis in host cells after GAS internalization. Evidence suggests that this may take place through extrinsic and intrinsic caspase pathways. The receptor-binding pathway and Fas-mediated apoptotic signaling pathway have been implicated in this process.[4]
See also
Todar's Online Textbook of Bacteriology
Streptococcal Pyrogenic Exotoxin A1
References
- 1 2 3 4 Brosnahan, A.J.; Schlievert, P.M. (December 2011). "Gram-positive bacterial superantigen outside-in signaling causes toxic shock syndrome: Superantigen outside-in signaling". The FEBS Journal. 278: 4649–67. PMC 3165073
 . PMID 21535475. doi:10.1111/j.1742-4658.2011.08151.x.
. PMID 21535475. doi:10.1111/j.1742-4658.2011.08151.x. - 1 2 3 4 5 Spaulding, A.R.; Salgado-Pabon, W.; Kohler, P.L.; Horswill, A.R.; Leung, D.Y.M.; Schlievert, P.M. "Staphylococcal and Streptococcal Superantigen Exotoxins". Clinical Microbiology Reviews. 26: 422–47. PMC 3719495
 . PMID 23824366. doi:10.1128/CMR.00104-12.
. PMID 23824366. doi:10.1128/CMR.00104-12. - 1 2 3 4 Llewelyn, M.; Cohen, J. (March 2002). "Superantigens: microbial agents that corrupt immunity". The Lancet Infectious Diseases. 2: 156–62. PMID 11944185. doi:10.1016/s1473-3099(02)00222-0.
- 1 2 3 4 5 6 7 Chiang-Ni, C.; Wu, J.-J. "Effects of Streptococcal Pyrogenic Exotoxin B on Pathogenesis of Streptococcus pyogenes". Journal of the Formosan Medical Association. 107: 677–85. PMID 18796357. doi:10.1016/S0929-6646(08)60112-6.
- 1 2 3 4 5 Nelson, Daniel C.; Garbe, Julia; Collin, Mattias (2011). "Cysteine proteinase SpeB from Streptococcus pyogenes – a potent modifier of immunologically important host and bacterial proteins". Biological Chemistry. 392 (12): 1077–88. PMID 22050223. doi:10.1515/bc.2011.208.
- 1 2 Brosnahan, Amanda J.; Schlievert, Patrick M. (2011-12-01). "Gram-positive bacterial superantigen outside-in signaling causes toxic shock syndrome". The FEBS Journal. 278 (23): 4649–4667. ISSN 1742-4658. PMC 3165073
 . PMID 21535475. doi:10.1111/j.1742-4658.2011.08151.x.
. PMID 21535475. doi:10.1111/j.1742-4658.2011.08151.x. - ↑ Li, H.; Llera, A.; Malchiodi, E. L.; Mariuzza, R. A. (1999-01-01). "The structural basis of T cell activation by superantigens". Annual Review of Immunology. 17: 435–466. ISSN 0732-0582. PMID 10358765. doi:10.1146/annurev.immunol.17.1.435.
- ↑ Tortora, Gerard; Funke, Berdell; Case, Christine (2013). Microbiology (11th ed.). Pearson. p. 439.
- ↑ Knöll H, Srámek J, Vrbová K, Gerlach D, Reichardt W, Köhler W (December 1991). "Scarlet fever and types of erythrogenic toxins produced by the infecting streptococcal strains". Zentralbl Bakteriol. 276 (1): 94–106. PMID 1789905. doi:10.1016/s0934-8840(11)80223-9.
- ↑ McShan, WM; Tang, YF; Ferretti, JJ (1997). "Bacteriophage T12 of Streptococcus pyogenes integrates into the gene encoding a serine tRNA". Molecular Microbiology. 23 (4): 719–28. PMID 9157243. doi:10.1046/j.1365-2958.1997.2591616.x.
- 1 2 3 4 5 6 Carroll, Ronan K.; Musser, James M. (2011-08-01). "From transcription to activation: how group A streptococcus, the flesh-eating pathogen, regulates SpeB cysteine protease production". Molecular Microbiology. 81 (3): 588–601. ISSN 1365-2958. PMID 21707787. doi:10.1111/j.1365-2958.2011.07709.x.
- 1 2 3 Nelson, Daniel C.; Garbe, Julia; Collin, Mattias (2011-12-01). "Cysteine proteinase SpeB from Streptococcus pyogenes - a potent modifier of immunologically important host and bacterial proteins". Biological Chemistry. 392 (12): 1077–1088. ISSN 1437-4315. PMID 22050223. doi:10.1515/BC.2011.208.
- ↑ "pyrogen". The Free Dictionary.
- 1 2 Chiang-Ni, Chuan; Wu, Jiunn-Jong. "Effects of Streptococcal Pyrogenic Exotoxin B on Pathogenesis of Streptococcus pyogenes". Journal of the Formosan Medical Association. 107 (9): 677–685. PMID 18796357. doi:10.1016/s0929-6646(08)60112-6.
- ↑ Boyd, E. Fidelma (2012-01-01). Szybalski, Małgorzata Łobocka and Wacław T., ed. Chapter 4 - Bacteriophage-Encoded Bacterial Virulence Factors and Phage–Pathogenicity Island Interactions. Bacteriophages, Part A. 82. Academic Press. pp. 91–118. doi:10.1016/b978-0-12-394621-8.00014-5.
- ↑ Hongmin Li; Andrea Llera; Emilio L. Malchiodi; Mariuzza, Roy A. (1999-01-01). "The Structural Basis of T Cell Activation by Superantigens". Annual Review of Immunology. 17 (1): 435–466. PMID 10358765. doi:10.1146/annurev.immunol.17.1.435.