Endothelial NOS
Endothelial NOS (eNOS), also known as nitric oxide synthase 3 (NOS3) or constitutive NOS (cNOS), is an enzyme that in humans is encoded by the NOS3 gene located in the 7q35-7q36 region of chromosome 7.[3] This enzyme is one of three isoforms that synthesize nitric oxide (NO), a small gaseous and lipophilic molecule that participates in several biological processes.[4][5] The other isoforms include neuronal nitric oxide synthase (nNOS), which is constitutively expressed in specific neurons of the brain[6] and inducible nitric oxide synthase (iNOS), whose expression is typically induced in inflammatory diseases.[7] eNOS is primarily responsible for the generation of NO in the vascular endothelium,[8] a monolayer of flat cells lining the interior surface of blood vessels, at the interface between circulating blood in the lumen and the remainder of the vessel wall.[9] NO produced by eNOS in the vascular endothelium plays crucial roles in regulating vascular tone, cellular proliferation, leukocyte adhesion, and platelet aggregation.[10] Therefore, a functional eNOS is essential for a healthy cardiovascular system.
Structure and catalytic activities
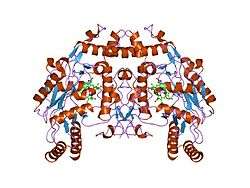
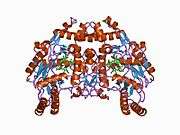
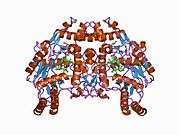
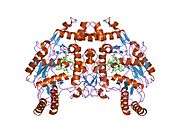
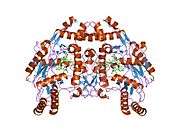
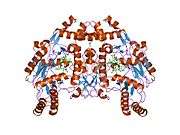
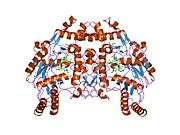
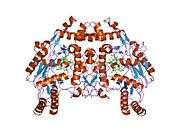
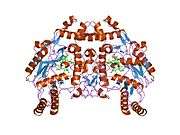
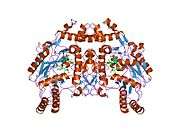
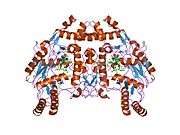
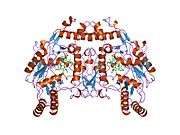
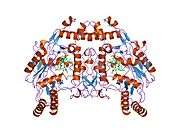
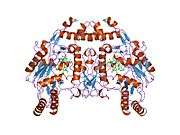
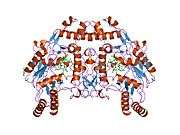
eNOS is a dimer containing two identical monomers of 134 kD constituted by a reductase domain, which displays binding sites for nicotinamide adenine dinucleotide phosphate (NADPH), flavin mononucleotide (FMN), and flavin adenine dinucleotide (FAD), and an oxidase domain, which displays binding sites for heme group, zinc, the cofactor tetrahydrobiopterin (BH4), and the substrate L-arginine.[11] The reductase domain is linked to the oxidase domain by a calmodulin-binding sequence.[12] In the vascular endothelium, NO is synthesized by eNOS from L-arginine and molecular oxygen, which binds to the heme group of eNOS, is reduced and finally incorporated into L- arginine to form NO and L-citrulline.[13][14] The binding of the cofactor BH4 is essential for eNOS to efficiently generate NO.[15] In the absence of this cofactor, eNOS shifts from a dimeric to a monomeric form, thus becoming uncoupled.[16] In this conformation, instead of synthesizing NO, eNOS produces superoxide anion, a highly reactive free radical with deleterious consequences to the cardiovascular system.[17][18]
Function
eNOS has a protective function in the cardiovascular system, which is attributed to NO production. Regulation of the vascular tone is one of the best known roles of NO in the cardiovascular system. Once produced in endothelial cells, NO diffuses across the vascular smooth muscle cell membranes and activates the enzyme soluble guanylate cyclase (sGC), which catalyzes the conversion of guanosine triphosphate into cyclic guanosine monophosphate (cGMP).[19] cGMP, in turn, activates protein kinase G (PKG), which promotes multiple phosphorylation of cellular targets lowering cellular Ca2+ concentrations and promoting vascular relaxation.[20] NO exerts antiproliferative effects by cGMP-dependent inhibiting Ca2+ influx or by directly inhibiting the activity of arginase and ornithine decarboxylase, decreasing the generation of polyamides required for DNA synthesis.[21][22] NO also has antithrombotic effects that result of its diffusion across platelet membrane and sGC activation, resulting in inhibition of platelet aggregation.[23] Moreover, NO affects leukocyte adhesion to the vascular endothelium by inhibiting the nuclear factor kappa B (NF-κB), which induces vascular endothelial expression of chemokines and adhesion molecules.[24] In addition to these functions, NO produced by eNOS has antioxidant properties as it reduces superoxide anion formation as a result of NO-induced increases in the expression of superoxide dismutase, an antioxidant enzyme that catalyzes the conversion of superoxide anion to hydrogen peroxide.[25] Furthermore, part of antioxidants properties of NO is attributable to up-regulation of heme-oxygenase-I and ferritin expression, which reduce superoxide anion concentrations in blood vessels.[26]
Regulation
eNOS expression and activity are controlled by multiple interconnected mechanisms of regulation present at the transcriptional, posttranscriptional, and posttranslational levels. Binding of transcription factors such as Sp1, Sp3, Ets-1, Elf-1, and YY1 to the NOS3 promoter and DNA methylation represents an important mechanism of transcriptional regulation.[27] Posttranscriptionally, eNOS is regulated by modifications of the primary transcript, mRNA stability, subcellular localization, and nucleocytoplasmatic transport.[28] Posttranslational modifications of eNOS include fatty acid acylation, protein-protein interactions, substrate, and co-factor availability, and degree of phosphorylation. Importantly, eNOS is attached by myristoylation and palmitoylation to caveolae, a pocket-like invagination on the membrane rich in cholesterol and sphingolipids.[29] With the binding of eNOS to caveolae, the enzyme is inactivated due to the strong and direct interaction of eNOS with caveolin-1.[30] The binding of calcium-activated calmodulin to eNOS displaces caveolin-1 and activates eNOS. Moreover, eNOS activation is dynamically regulated by multiple phosphorylation sites at tyrosine, serine, and threonine residues.[11]
Clinical significance
Impaired NO production is involved in the pathogenesis of several diseases such as hypertension, preeclampsia, diabetes mellitus, obesity, erectile dysfunction, and migraine. In this regard, a large number of studies showed that polymorphisms in NOS3 gene affect the susceptibility to these diseases. Although NOS3 is a highly polymorphic gene, three genetic polymorphisms in this gene have been widely studied: the single nucleotide polymorphisms (SNPs) g.-786T>C (where "g." denotes genomic change which results in a Glu298Asp change in the coded protein), located in NOS3 promoter and in exon 7, respectively, and the variable number of tandem repeats (VNTR) characterized by 27 bp repeat in intron 4.[31] The C allele for the g.-786T>C polymorphism, which results in reduced eNOS expression and NO production,[32] was associated with increased risk for hypertension,[33] preeclampsia,[34] diabetic nephropathy,[35] and retinopathy,[36] migraine,[37] and erectile dysfunction,[38] The presence of ‘Asp’ allele for the Glu298Asp polymorphism reduces eNOS activity,[39] and was associated with higher susceptibility to hypertension,[40][41] preeclampsia,[42] diabetes mellitus,[43] migraine,[37] and erectile dysfunction.[44][45] The VNTR in intron 4 affects eNOS expression,[46] and the susceptibility to hypertension,[33] preeclampsia,[34] obesity,[47] and diabetes mellitus.[43] Growing evidence supports the association of diseases with NOS3 haplotypes (combination of alleles in close proximity, within a DNA block). This approach may be more informative than the analysis of genetic polymorphisms one by one.[48] Haplotypes including the SNPs g.-786T>C and Glu298Asp and the VNTR in intron 4 affected the susceptibility to hypertension,[49][50][51][52] preeclampsia,[53] and hypertension in diabetic subjects,[54] NOS3 variants may also affect the responses to drugs that affect NO signaling, such as statins, angiotensin-converting enzyme inhibitors (ACEi) and phosphodiesterase type 5 (PDE-5) inhibitors (PDE5i). Statin treatment was more effective in increasing NO bioavailability in subjects carrying the CC genotype for the g.-786T>C polymorphism than in TT carriers.[55][56] Hypertensive patients carrying the TC/CC genotypes and the C allele for the g.-786T>C polymorphism showed better antihypertensive responses to ACEi enalapril.[57] Likewise, patients with erectile dysfunction carrying the C allele for g.-786T>C polymorphism showed better responses to PDE-5 inhibitor sildenafil.[58][59] Together, these studies suggest that statins, ACEi and PDE-5 inhibitors may restore an impaired NO production in subjects carrying the variant allele/genotype for g.-786T>C NOS3 polymorphism, thus attenuating the cardiovascular risk. In addition to analysis of genetic polymorphisms individually, haplotypes including the SNPs g.-786T>C and Glu298Asp and the VNTR in intron 4 were showen to affect the responses to sildenafil in patients with erectile dysfunction.[58]
References
- ↑ "Human PubMed Reference:".
- ↑ "Mouse PubMed Reference:".
- ↑ Marsden PA, Schappert KT, Chen HS, Flowers M, Sundell CL, Wilcox JN, Lamas S, Michel T (August 1992). "Molecular cloning and characterization of human endothelial nitric oxide synthase". FEBS Lett. 307 (3): 287–93. PMID 1379542. doi:10.1016/0014-5793(92)80697-F.
- ↑ Cockcroft JR (Dec 2005). "Exploring vascular benefits of endothelium-derived nitric oxide". American Journal of Hypertension. 18 (12 Pt 2): 177S–183S. PMID 16373196. doi:10.1016/j.amjhyper.2005.09.001.
- ↑ Villanueva C, Giulivi C (Aug 2010). "Subcellular and cellular locations of nitric oxide synthase isoforms as determinants of health and disease". Free Radical Biology & Medicine. 49 (3): 307–16. PMC 2900489
 . PMID 20388537. doi:10.1016/j.freeradbiomed.2010.04.004.
. PMID 20388537. doi:10.1016/j.freeradbiomed.2010.04.004. - ↑ Förstermann U, Sessa WC (Apr 2012). "Nitric oxide synthases: regulation and function". European Heart Journal. 33 (7): 829–37, 837a–837d. PMC 3345541
 . PMID 21890489. doi:10.1093/eurheartj/ehr304.
. PMID 21890489. doi:10.1093/eurheartj/ehr304. - ↑ Oliveira-Paula GH, Lacchini R, Tanus-Santos JE (Feb 2014). "Inducible nitric oxide synthase as a possible target in hypertension". Current Drug Targets. 15 (2): 164–74. PMID 24102471. doi:10.2174/13894501113146660227.
- ↑ Fish JE, Marsden PA (Jan 2006). "Endothelial nitric oxide synthase: insight into cell-specific gene regulation in the vascular endothelium". Cellular and Molecular Life Sciences. 63 (2): 144–62. PMID 16416260. doi:10.1007/s00018-005-5421-8.
- ↑ Sumpio BE, Riley JT, Dardik A (Dec 2002). "Cells in focus: endothelial cell". The International Journal of Biochemistry & Cell Biology. 34 (12): 1508–12. PMID 12379270. doi:10.1016/s1357-2725(02)00075-4.
- ↑ Förstermann U, Münzel T (Apr 2006). "Endothelial nitric oxide synthase in vascular disease: from marvel to menace". Circulation. 113 (13): 1708–14. PMID 16585403. doi:10.1161/CIRCULATIONAHA.105.602532.
- 1 2 Qian J, Fulton D (2013). "Post-translational regulation of endothelial nitric oxide synthase in vascular endothelium". Frontiers in Physiology. 4: 347. PMC 3861784
 . PMID 24379783. doi:10.3389/fphys.2013.00347.
. PMID 24379783. doi:10.3389/fphys.2013.00347. - ↑ Alderton WK, Cooper CE, Knowles RG (Aug 2001). "Nitric oxide synthases: structure, function and inhibition". The Biochemical Journal. 357 (Pt 3): 593–615. PMC 1221991
 . PMID 11463332. doi:10.1042/bj3570593.
. PMID 11463332. doi:10.1042/bj3570593. - ↑ Fleming I, Busse R (Aug 1999). "Signal transduction of eNOS activation". Cardiovascular Research. 43 (3): 532–41. PMID 10690325. doi:10.1016/s0008-6363(99)00094-2.
- ↑ Verhaar MC, Westerweel PE, van Zonneveld AJ, Rabelink TJ (May 2004). "Free radical production by dysfunctional eNOS". Heart. 90 (5): 494–5. PMC 1768213
 . PMID 15084540. doi:10.1136/hrt.2003.029405.
. PMID 15084540. doi:10.1136/hrt.2003.029405. - ↑ Dudzinski DM, Igarashi J, Greif D, Michel T (2006). "The regulation and pharmacology of endothelial nitric oxide synthase". Annual Review of Pharmacology and Toxicology. 46: 235–76. PMID 16402905. doi:10.1146/annurev.pharmtox.44.101802.121844.
- ↑ Maron BA, Michel T (2012). "Subcellular localization of oxidants and redox modulation of endothelial nitric oxide synthase". Circulation Journal. 76 (11): 2497–512. PMID 23075817. doi:10.1253/circj.cj-12-1207.
- ↑ Albrecht EW, Stegeman CA, Heeringa P, Henning RH, van Goor H (Jan 2003). "Protective role of endothelial nitric oxide synthase". The Journal of Pathology. 199 (1): 8–17. PMID 12474221. doi:10.1002/path.1250.
- ↑ Luo S, Lei H, Qin H, Xia Y (2014). "Molecular mechanisms of endothelial NO synthase uncoupling". Current Pharmaceutical Design. 20 (22): 3548–53. PMID 24180388. doi:10.2174/13816128113196660746.
- ↑ Denninger JW, Marletta MA (May 1999). "Guanylate cyclase and the .NO/cGMP signaling pathway". Biochimica et Biophysica Acta. 1411 (2–3): 334–50. PMID 10320667. doi:10.1016/s0005-2728(99)00024-9.
- ↑ Surks HK, Mochizuki N, Kasai Y, Georgescu SP, Tang KM, Ito M, Lincoln TM, Mendelsohn ME (Nov 1999). "Regulation of myosin phosphatase by a specific interaction with cGMP- dependent protein kinase Ialpha". Science. 286 (5444): 1583–7. PMID 10567269. doi:10.1126/science.286.5444.1583.
- ↑ Cornwell TL, Arnold E, Boerth NJ, Lincoln TM (Nov 1994). "Inhibition of smooth muscle cell growth by nitric oxide and activation of cAMP-dependent protein kinase by cGMP". The American Journal of Physiology. 267 (5 Pt 1): C1405–13. PMID 7977701.
- ↑ Ignarro LJ, Buga GM, Wei LH, Bauer PM, Wu G, del Soldato P (Mar 2001). "Role of the arginine-nitric oxide pathway in the regulation of vascular smooth muscle cell proliferation". Proceedings of the National Academy of Sciences of the United States of America. 98 (7): 4202–8. PMC 31203
 . PMID 11259671. doi:10.1073/pnas.071054698.
. PMID 11259671. doi:10.1073/pnas.071054698. - ↑ Walford G, Loscalzo J (Oct 2003). "Nitric oxide in vascular biology". Journal of Thrombosis and Haemostasis. 1 (10): 2112–8. PMID 14521592. doi:10.1046/j.1538-7836.2003.00345.x.
- ↑ Chen F, Castranova V, Shi X, Demers LM (Jan 1999). "New insights into the role of nuclear factor-kappaB, a ubiquitous transcription factor in the initiation of diseases". Clinical Chemistry. 45 (1): 7–17. PMID 9895331.
- ↑ Fukai T, Siegfried MR, Ushio-Fukai M, Cheng Y, Kojda G, Harrison DG (Jun 2000). "Regulation of the vascular extracellular superoxide dismutase by nitric oxide and exercise training". The Journal of Clinical Investigation. 105 (11): 1631–9. PMC 300857
 . PMID 10841522. doi:10.1172/JCI9551.
. PMID 10841522. doi:10.1172/JCI9551. - ↑ Balla G, Jacob HS, Balla J, Rosenberg M, Nath K, Apple F, Eaton JW, Vercellotti GM (Sep 1992). "Ferritin: a cytoprotective antioxidant strategem of endothelium". The Journal of Biological Chemistry. 267 (25): 18148–53. PMID 1517245.
- ↑ Karantzoulis-Fegaras F, Antoniou H, Lai SL, Kulkarni G, D'Abreo C, Wong GK, Miller TL, Chan Y, Atkins J, Wang Y, Marsden PA (Jan 1999). "Characterization of the human endothelial nitric-oxide synthase promoter". The Journal of Biological Chemistry. 274 (5): 3076–93. PMID 9915847. doi:10.1074/jbc.274.5.3076.
- ↑ Searles CD (Nov 2006). "Transcriptional and posttranscriptional regulation of endothelial nitric oxide synthase expression". American Journal of Physiology. Cell Physiology. 291 (5): C803–16. PMID 16738003. doi:10.1152/ajpcell.00457.2005.
- ↑ Lisanti MP, Scherer PE, Tang Z, Sargiacomo M (Jul 1994). "Caveolae, caveolin and caveolin-rich membrane domains: a signalling hypothesis". Trends in Cell Biology. 4 (7): 231–5. PMID 14731661. doi:10.1016/0962-8924(94)90114-7.
- ↑ Ju H, Zou R, Venema VJ, Venema RC (Jul 1997). "Direct interaction of endothelial nitric-oxide synthase and caveolin-1 inhibits synthase activity". The Journal of Biological Chemistry. 272 (30): 18522–5. PMID 9228013. doi:10.1074/jbc.272.30.18522.
- ↑ Lacchini R, Silva PS, Tanus-Santos JE (May 2010). "A pharmacogenetics-based approach to reduce cardiovascular mortality with the prophylactic use of statins". Basic & Clinical Pharmacology & Toxicology. 106 (5): 357–61. PMID 20210789. doi:10.1111/j.1742-7843.2010.00551.x.
- ↑ Nakayama M, Yasue H, Yoshimura M, Shimasaki Y, Kugiyama K, Ogawa H, Motoyama T, Saito Y, Ogawa Y, Miyamoto Y, Nakao K (Jun 1999). "T-786→C mutation in the 5'-flanking region of the endothelial nitric oxide synthase gene is associated with coronary spasm". Circulation. 99 (22): 2864–70. PMID 10359729. doi:10.1161/01.cir.99.22.2864.
- 1 2 Niu W, Qi Y (2011). "An updated meta-analysis of endothelial nitric oxide synthase gene: three well-characterized polymorphisms with hypertension". PLOS ONE. 6 (9): e24266. PMC 3166328
 . PMID 21912683. doi:10.1371/journal.pone.0024266.
. PMID 21912683. doi:10.1371/journal.pone.0024266. - 1 2 Dai B, Liu T, Zhang B, Zhang X, Wang Z (Apr 2013). "The polymorphism for endothelial nitric oxide synthase gene, the level of nitric oxide and the risk for pre-eclampsia: a meta-analysis". Gene. 519 (1): 187–93. PMID 23375994. doi:10.1016/j.gene.2013.01.004.
- ↑ Shoukry A, Shalaby SM, Abdelazim S, Abdelazim M, Ramadan A, Ismail MI, Fouad M (Jun 2012). "Endothelial nitric oxide synthase gene polymorphisms and the risk of diabetic nephropathy in type 2 diabetes mellitus". Genetic Testing and Molecular Biomarkers. 16 (6): 574–9. PMID 22313046. doi:10.1089/gtmb.2011.0218.
- ↑ Taverna MJ, Elgrably F, Selmi H, Selam JL, Slama G (Aug 2005). "The T-786C and C774T endothelial nitric oxide synthase gene polymorphisms independently affect the onset pattern of severe diabetic retinopathy". Nitric Oxide. 13 (1): 88–92. PMID 15890549. doi:10.1016/j.niox.2005.04.004.
- 1 2 Eröz R, Bahadir A, Dikici S, Tasdemir S (Sep 2014). "Association of endothelial nitric oxide synthase gene polymorphisms (894G/T, -786T/C, G10T) and clinical findings in patients with migraine". Neuromolecular Medicine. 16 (3): 587–93. PMID 24845269. doi:10.1007/s12017-014-8311-0.
- ↑ Safarinejad MR, Khoshdel A, Shekarchi B, Taghva A, Safarinejad S (Jun 2011). "Association of the T-786C, G894T and 4a/4b polymorphisms of the endothelial nitric oxide synthase gene with vasculogenic erectile dysfunction in Iranian subjects". BJU International. 107 (12): 1994–2001. PMID 20955262. doi:10.1111/j.1464-410X.2010.09755.x.
- ↑ Joshi MS, Mineo C, Shaul PW, Bauer JA (Sep 2007). "Biochemical consequences of the NOS3 Glu298Asp variation in human endothelium: altered caveolar localization and impaired response to shear". FASEB Journal. 21 (11): 2655–63. PMID 17449720. doi:10.1096/fj.06-7088com.
- ↑ Liu J, Wang L, Liu Y, Wang Z, Li M, Zhang B, Wang H, Liu K, Wen S (Mar 2015). "The association between endothelial nitric oxide synthase gene G894T polymorphism and hypertension in Han Chinese: a case-control study and an updated meta-analysis". Annals of Human Biology. 42 (2): 184–94. PMID 24846690. doi:10.3109/03014460.2014.911958.
- ↑ Pereira TV, Rudnicki M, Cheung BM, Baum L, Yamada Y, Oliveira PS, Pereira AC, Krieger JE (Sep 2007). "Three endothelial nitric oxide (NOS3) gene polymorphisms in hypertensive and normotensive individuals: meta-analysis of 53 studies reveals evidence of publication bias". Journal of Hypertension. 25 (9): 1763–74. PMID 17762636. doi:10.1097/HJH.0b013e3281de740d.
- ↑ Serrano NC, Casas JP, Díaz LA, Páez C, Mesa CM, Cifuentes R, Monterrosa A, Bautista A, Hawe E, Hingorani AD, Vallance P, López-Jaramillo P (Nov 2004). "Endothelial NO synthase genotype and risk of preeclampsia: a multicenter case-control study". Hypertension. 44 (5): 702–7. PMID 15364897. doi:10.1161/01.HYP.0000143483.66701.ec.
- 1 2 Jia Z, Zhang X, Kang S, Wu Y (2013). "Association of endothelial nitric oxide synthase gene polymorphisms with type 2 diabetes mellitus: a meta-analysis". Endocrine Journal. 60 (7): 893–901. PMID 23563728. doi:10.1507/endocrj.ej12-0463.
- ↑ Lee YC, Huang SP, Liu CC, Yang YH, Yeh HC, Li WM, Wu WJ, Wang CJ, Juan YS, Huang CN, Hour TC, Chang CF, Huang CH (Mar 2012). "The association of eNOS G894T polymorphism with metabolic syndrome and erectile dysfunction". The Journal of Sexual Medicine. 9 (3): 837–43. PMID 22304542. doi:10.1111/j.1743-6109.2011.02588.x.
- ↑ Hermans MP, Ahn SA, Rousseau MF (Jul 2012). "eNOS [Glu298Asp] polymorphism, erectile function and ocular pressure in type 2 diabetes". European Journal of Clinical Investigation. 42 (7): 729–37. PMID 22224829. doi:10.1111/j.1365-2362.2011.02638.x.
- ↑ Zhang MX, Zhang C, Shen YH, Wang J, Li XN, Chen L, Zhang Y, Coselli JS, Wang XL (Sep 2008). "Effect of 27nt small RNA on endothelial nitric-oxide synthase expression". Molecular Biology of the Cell. 19 (9): 3997–4005. PMC 2526692
 . PMID 18614799. doi:10.1091/mbc.E07-11-1186.
. PMID 18614799. doi:10.1091/mbc.E07-11-1186. - ↑ Souza-Costa DC, Belo VA, Silva PS, Sertorio JT, Metzger IF, Lanna CM, Machado MA, Tanus-Santos JE (Mar 2011). "eNOS haplotype associated with hypertension in obese children and adolescents". International Journal of Obesity. 35 (3): 387–92. PMID 20661250. doi:10.1038/ijo.2010.146.
- ↑ Crawford DC, Nickerson DA (2005). "Definition and clinical importance of haplotypes". Annual Review of Medicine. 56: 303–20. PMID 15660514. doi:10.1146/annurev.med.56.082103.104540.
- ↑ Sandrim VC, Coelho EB, Nobre F, Arado GM, Lanchote VL, Tanus-Santos JE (Jun 2006). "Susceptible and protective eNOS haplotypes in hypertensive black and white subjects". Atherosclerosis. 186 (2): 428–32. PMID 16168996. doi:10.1016/j.atherosclerosis.2005.08.003.
- ↑ Sandrim VC, de Syllos RW, Lisboa HR, Tres GS, Tanus-Santos JE (Nov 2006). "Endothelial nitric oxide synthase haplotypes affect the susceptibility to hypertension in patients with type 2 diabetes mellitus". Atherosclerosis. 189 (1): 241–6. PMID 16427644. doi:10.1016/j.atherosclerosis.2005.12.011.
- ↑ Sandrim VC, Yugar-Toledo JC, Desta Z, Flockhart DA, Moreno H, Tanus-Santos JE (Dec 2006). "Endothelial nitric oxide synthase haplotypes are related to blood pressure elevation, but not to resistance to antihypertensive drug therapy". Journal of Hypertension. 24 (12): 2393–7. PMID 17082721. doi:10.1097/01.hjh.0000251899.47626.4f.
- ↑ Vasconcellos V, Lacchini R, Jacob-Ferreira AL, Sales ML, Ferreira-Sae MC, Schreiber R, Nadruz W, Tanus-Santos JE (Apr 2010). "Endothelial nitric oxide synthase haplotypes associated with hypertension do not predispose to cardiac hypertrophy". DNA and Cell Biology. 29 (4): 171–6. PMID 20070154. doi:10.1089/dna.2009.0955.
- ↑ Sandrim VC, Palei AC, Sertorio JT, Cavalli RC, Duarte G, Tanus-Santos JE (Jul 2010). "Effects of eNOS polymorphisms on nitric oxide formation in healthy pregnancy and in pre-eclampsia". Molecular Human Reproduction. 16 (7): 506–10. PMID 20457799. doi:10.1093/molehr/gaq030.
- ↑ de Syllos RW, Sandrim VC, Lisboa HR, Tres GS, Tanus-Santos JE (Dec 2006). "Endothelial nitric oxide synthase genotype and haplotype are not associated with diabetic retinopathy in diabetes type 2 patients". Nitric Oxide. 15 (4): 417–22. PMID 16581274. doi:10.1016/j.niox.2006.02.002.
- ↑ Nagassaki S, Sertório JT, Metzger IF, Bem AF, Rocha JB, Tanus-Santos JE (Oct 2006). "eNOS gene T-786C polymorphism modulates atorvastatin-induced increase in blood nitrite". Free Radical Biology & Medicine. 41 (7): 1044–9. PMID 16962929. doi:10.1016/j.freeradbiomed.2006.04.026.
- ↑ Andrade VL, Sertório JT, Eleuterio NM, Tanus-Santos JE, Fernandes KS, Sandrim VC (Sep 2013). "Simvastatin treatment increases nitrite levels in obese women: modulation by T(-786)C polymorphism of eNOS". Nitric Oxide. 33: 83–7. PMID 23876348. doi:10.1016/j.niox.2013.07.005.
- ↑ Silva PS, Fontana V, Luizon MR, Lacchini R, Silva WA, Biagi C, Tanus-Santos JE (Feb 2013). "eNOS and BDKRB2 genotypes affect the antihypertensive responses to enalapril". European Journal of Clinical Pharmacology. 69 (2): 167–77. PMID 22706620. doi:10.1007/s00228-012-1326-2.
- 1 2 Muniz JJ, Lacchini R, Rinaldi TO, Nobre YT, Cologna AJ, Martins AC, Tanus-Santos JE (Apr 2013). "Endothelial nitric oxide synthase genotypes and haplotypes modify the responses to sildenafil in patients with erectile dysfunction". The Pharmacogenomics Journal. 13 (2): 189–96. PMID 22064666. doi:10.1038/tpj.2011.49.
- ↑ Lacchini R, Tanus-Santos JE (Aug 2014). "Pharmacogenetics of erectile dysfunction: navigating into uncharted waters". Pharmacogenomics. 15 (11): 1519–38. PMID 25303302. doi:10.2217/pgs.14.110.
Further reading
- de la Monte SM, Lu BX, Sohn YK, Etienne D, Kraft J, Ganju N, Wands JR (2000). "Aberrant expression of nitric oxide synthase III in Alzheimer's disease: relevance to cerebral vasculopathy and neurodegeneration". Neurobiology of Aging. 21 (2): 309–19. PMID 10867216. doi:10.1016/S0197-4580(99)00108-6.
- Shaul PW (2002). "Regulation of endothelial nitric oxide synthase: location, location, location". Annual Review of Physiology. 64: 749–74. PMID 11826287. doi:10.1146/annurev.physiol.64.081501.155952.
- Wu KK (May 2002). "Regulation of endothelial nitric oxide synthase activity and gene expression". Annals of the New York Academy of Sciences. 962: 122–30. PMID 12076969. doi:10.1111/j.1749-6632.2002.tb04062.x.
- Alp NJ, Channon KM (Mar 2004). "Regulation of endothelial nitric oxide synthase by tetrahydrobiopterin in vascular disease". Arteriosclerosis, Thrombosis, and Vascular Biology. 24 (3): 413–20. PMID 14656731. doi:10.1161/01.ATV.0000110785.96039.f6.
- Tai SC, Robb GB, Marsden PA (Mar 2004). "Endothelial nitric oxide synthase: a new paradigm for gene regulation in the injured blood vessel". Arteriosclerosis, Thrombosis, and Vascular Biology. 24 (3): 405–12. PMID 14656742. doi:10.1161/01.ATV.0000109171.50229.33.
- Kawashima S, Yokoyama M (Jun 2004). "Dysfunction of endothelial nitric oxide synthase and atherosclerosis". Arteriosclerosis, Thrombosis, and Vascular Biology. 24 (6): 998–1005. PMID 15001455. doi:10.1161/01.ATV.0000125114.88079.96.
- Duda DG, Fukumura D, Jain RK (Apr 2004). "Role of eNOS in neovascularization: NO for endothelial progenitor cells". Trends in Molecular Medicine. 10 (4): 143–5. PMID 15162796. doi:10.1016/j.molmed.2004.02.001.