Endocrine system
| Endocrine system | |
|---|---|
 Main glands of the endocrine system. Note that the thymus is no longer considered part of the endocrine system, as it does not produce hormones. | |
| Details | |
| Identifiers | |
| Latin | Systema endocrinum |
| FMA | 9668 |
The endocrine system is the collection of glands of an organism that secrete hormones directly into the circulatory system to be carried towards distant target organs. The phenomenon of biochemical processes' serving to regulate distant tissues by means of secretions directly into the circulatory system is called endocrine signaling. The major endocrine glands include the pineal gland, pituitary gland, pancreas, ovaries, testes, thyroid gland, parathyroid gland, and adrenal glands. The endocrine system is in contrast to the exocrine system, which secretes its hormones to the outside of the body using ducts. The endocrine system is an information signal system like the nervous system, yet its effects and mechanism are classifiably different. The endocrine system's effects are slow to initiate, and prolonged in their response, lasting from a few hours up to weeks. The nervous system sends information very quickly, and responses are generally short lived. In vertebrates, the hypothalamus is the neural control center for all endocrine systems. The field of study dealing with the endocrine system and its disorders is endocrinology, a branch of internal medicine.[1] Special features of endocrine glands are, in general, their ductless nature, their vascularity, and commonly the presence of intracellular vacuoles or granules that store their hormones. In contrast, exocrine glands, such as salivary glands, sweat glands, and glands within the gastrointestinal tract, tend to be much less vascular and have ducts or a hollow lumen.
In addition to the specialized endocrine organs mentioned above, many other organs that are part of other body systems, such as bone, kidney, liver, heart and gonads, have secondary endocrine functions. For example, the kidney secretes endocrine hormones such as erythropoietin and renin. Hormones can consist of either amino acid complexes, steroids, eicosanoids, leukotrienes, or prostaglandins.[1]
A number of glands that signal each other in sequence are usually referred to as an axis, for example, the hypothalamic-pituitary-adrenal axis.
As opposed to endocrine factors that travel considerably longer distances via the circulatory system, other signaling molecules, such as paracrine factors involved in paracrine signalling diffuse over a relatively short distance.
The word endocrine derives from the Greek words ἐνδο- endo- "inside, within," and κρίνειν krinein "to separate, distinguish".
Endocrine organs and known secreted hormones

Hypothalamus
| Secreted hormone | Abbreviation | Produced by | Effect |
|---|---|---|---|
| Thyrotropin-releasing hormone | TRH | Parvocellular neurosecretory neurons | Stimulate thyroid-stimulating hormone (TSH) release from anterior pituitary (primarily) |
| Dopamine (Prolactin-inhibiting hormone) |
DA or PIH | Dopamine neurons of the arcuate nucleus | Inhibit prolactin released from anterior pituitary |
| Growth hormone-releasing hormone | GHRH | Neuroendocrine neurons of the Arcuate nucleus | Stimulate Growth hormone (GH) release from anterior pituitary |
| Somatostatin (growth hormone-inhibiting hormone) |
SS, GHIH, or SRIF | Neuroendocrine cells of the Periventricular nucleus | Inhibit Growth hormone release from anterior pituitary Inhibit thyroid-stimulating hormone (TSH) release from anterior pituitary |
| Gonadotropin-releasing hormone | GnRH or LHRH | Neuroendocrine cells of the Preoptic area | Stimulate follicle-stimulating hormone (FSH) release from anterior pituitary Stimulate luteinizing hormone (LH) release from anterior pituitary |
| Corticotropin-releasing hormone | CRH or CRF | Parvocellular neurosecretory neurons of the Paraventricular Nucleus | Stimulate adrenocorticotropic hormone (ACTH) release from anterior pituitary |
| Vasopressin (antidiuretic hormone) |
ADH or AVP or VP | Parvocellular neurosecretory neurons, Magnocellular neurosecretory neurons of the Paraventricular nucleus and Supraoptic nucleus | Increases water permeability in the distal convoluted tubule and collecting duct of nephrons, thus promoting water reabsorption and increasing blood volume |
Pineal body (epiphysis)
| Secreted hormone | From cells | Effect |
|---|---|---|
| Melatonin | Pinealocytes | Antioxidant Monitors the circadian rhythm including induction of drowsiness and lowering of the core body temperature |
Pituitary gland (hypophysis)
The pituitary gland (or hypophysis) is an endocrine gland about the size of a pea and weighing 0.5 grams (0.018 oz) in humans. It is a protrusion off the bottom of the hypothalamus at the base of the brain, and rests in a small, bony cavity (sella turcica) covered by a dural fold (diaphragma sellae). The pituitary is functionally connected to the hypothalamus by the median eminence via a small tube called the infundibular stem or pituitary stalk.[2] The anterior pituitary (adenohypophysis) is connected to the hypothalamus via the hypothalamo–hypophyseal portal vessels, which allows for quicker and more efficient communication between the hypothalamus and the pituitary.[3]
Anterior pituitary lobe (adenohypophysis)
| Secreted hormone | Abbreviation | From cells | Effect |
|---|---|---|---|
| Growth hormone (somatotropin) |
GH | Somatotrophs | Stimulates growth and cell reproduction Stimulates Insulin-like growth factor 1 release from liver |
| Thyroid-stimulating hormone (thyrotropin) |
TSH | Thyrotrophs | Stimulates thyroxine (T4) and triiodothyronine (T3) synthesis and release from thyroid gland Stimulates iodine absorption by thyroid gland |
| Adrenocorticotropic hormone (corticotropin) |
ACTH | Corticotrophs | Stimulates corticosteroid (glucocorticoid and mineralcorticoid) and androgen synthesis and release from adrenocortical cells |
| Beta-endorphin | – | Corticotrophs | Inhibits perception of pain |
| Follicle-stimulating hormone | FSH | Gonadotrophs | In females: Stimulates maturation of ovarian follicles in ovary In males: Stimulates maturation of seminiferous tubules In males: Stimulates spermatogenesis In males: Stimulates production of androgen-binding protein from Sertoli cells of the testes |
| Luteinizing hormone | LH | Gonadotrophs | In females: Stimulates ovulation In females: Stimulates formation of corpus luteum In males: Stimulates testosterone synthesis from Leydig cells (interstitial cells) |
| Prolactin | PRL | Lactotrophs | Stimulates milk synthesis and release from mammary glands Mediates sexual gratification |
| Melanocyte-stimulating hormone | MSH | Melanotropes in the Pars intermedia of the Anterior Pituitary | Stimulates melanin synthesis and release from skin/hair melanocytes |
Posterior pituitary lobe (neurohypophysis)
| Stored hormone | Abbreviation | From cells | Effect |
|---|---|---|---|
| Oxytocin | OX or OXT | Magnocellular neurosecretory cells | In females: uterine contraction during birthing, lactation (letdown reflex) when nursing |
| Vasopressin (antidiuretic hormone) |
ADH or AVP | Parvocellular neurosecretory neurons | Increases water permeability in the distal convoluted tubule and collecting duct of nephrons, thus promoting water reabsorption and increasing blood volume |
Oxytocin and anti-diuretic hormone are not secreted in the posterior lobe, merely stored.
Thyroid
| Secreted hormone | Abbreviation | From cells | Effect |
|---|---|---|---|
| Triiodothyronine | T3 | Thyroid epithelial cell | (More potent form of thyroid hormone) Stimulates body oxygen and energy consumption, thereby increasing the basal metabolic rate Stimulates RNA polymerase I and II, thereby promoting protein synthesis |
| Thyroxine (tetraiodothyronine) |
T4 | Thyroid epithelial cells | (Less active form of thyroid hormone) (Acts as a prohormone to triiodothyronine) Stimulates body oxygen and energy consumption, thereby increasing the basal metabolic rate Stimulates RNA polymerase I and II, thereby promoting protein synthesis |
| Calcitonin | Parafollicular cells | Stimulates osteoblasts and thus bone construction Inhibits Ca2+ release from bone, thereby reducing blood Ca2+ |
Digestive system
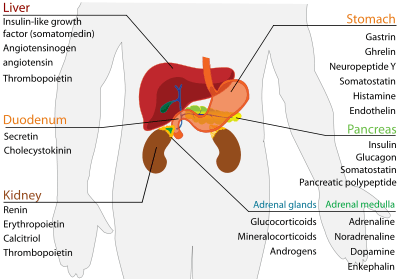
Stomach
| Secreted hormone | Abbreviation | From cells | Effect |
|---|---|---|---|
| Gastrin (Primarily) | G cells | Secretion of gastric acid by parietal cells | |
| Ghrelin | P/D1 cells | Stimulate appetite. | |
| Neuropeptide Y | NPY | Increased food intake and decreased physical activity. It can be associated with obesity. | |
| Somatostatin | D cells | Suppress release of gastrin, cholecystokinin (CCK), secretin, motilin, vasoactive intestinal peptide (VIP), gastric inhibitory polypeptide (GIP), enteroglucagon
Lowers rate of gastric emptying Reduces smooth muscle contractions and blood flow within the intestine.[4] | |
| Histamine | ECL cells | stimulate gastric acid secretion | |
| Endothelin | X cells | Smooth muscle contraction of stomach[5] |
Duodenum (small intestine)
| Secreted hormone | From cells | Effect |
|---|---|---|
| Secretin | S cells | Secretion of bicarbonate from liver, pancreas and duodenal Brunner's glands
Enhances effects of cholecystokinin, stops production of gastric juice |
| Cholecystokinin | I cells | Release of digestive enzymes from pancreas
Release of bile from gallbladder, hunger suppressant |
Liver
| Secreted hormone | Abbreviation | From cells | Effect |
|---|---|---|---|
| Insulin-like growth factor (or somatomedin) (Primarily) | IGF | Hepatocytes | insulin-like effects
regulate cell growth and development |
| Angiotensinogen and angiotensin | Hepatocytes | vasoconstriction
release of aldosterone from adrenal cortex dipsogen. | |
| Thrombopoietin | THPO | Hepatocytes | stimulates megakaryocytes to produce platelets[6] |
| Hepcidin | Hepatocytes | inhibits intestinal iron absorption and iron release by macrophages |
Pancreas
The pancreas is a mixocrine gland and it secretes both enzymes and hormones.
| Secreted hormone | From cells | Effect |
|---|---|---|
| Insulin (Primarily) | β Islet cells | Intake of glucose, glycogenesis and glycolysis in liver and muscle from blood.
Intake of lipids and synthesis of triglycerides in adipocytes. Other anabolic effects |
| Glucagon (Also Primarily) | α Islet cells | Glycogenolysis and gluconeogenesis in liver.
Increases blood glucose level. |
| Somatostatin | δ Islet cells | Inhibit release of insulin[7]
Inhibit release of glucagon[7] Suppress the exocrine secretory action of pancreas. |
| Pancreatic polypeptide | PP cells | Self regulate the pancreas secretion activities and effect the hepatic glycogen levels. |
Kidney
| Secreted hormone | From cells | Effect |
|---|---|---|
| Renin (Primarily) | Juxtaglomerular cells | Activates the renin-angiotensin system by producing angiotensin I of angiotensinogen |
| Erythropoietin (EPO) | Extraglomerular mesangial cells | Stimulate erythrocyte production |
| Calcitriol (1,25-dihydroxyvitamin D3) | Proximal tubule cells | Active form of vitamin D3
Increase absorption of calcium and phosphate from gastrointestinal tract and kidneys inhibit release of PTH |
| Thrombopoietin | stimulates megakaryocytes to produce platelets[6] |
Adrenal glands
Adrenal cortex
| Secreted hormone | From cells | Effect |
|---|---|---|
| Glucocorticoids (chiefly cortisol) | zona fasciculata and zona reticularis cells | Stimulates gluconeogenesis Stimulates fat breakdown in adipose tissue Inhibits protein synthesis Inhibits glucose uptake in muscle and adipose tissue Inhibits immunological responses (immunosuppressive) Inhibits inflammatory responses (anti-inflammatory) |
| Mineralocorticoids (chiefly aldosterone) | Zona glomerulosa cells | Stimulates active sodium reabsorption in kidneys Stimulates passive water reabsorption in kidneys, thus increasing blood volume and blood pressure Stimulates potassium and H+ secretion into nephron of kidney and subsequent excretion |
| Androgens (including DHEA and testosterone) | Zona fasciculata and Zona reticularis cells | In males: Relatively small effect compared to androgens from testes In females: masculinizing effects |
Adrenal medulla
| Secreted hormone | From cells | Effect |
|---|---|---|
| Adrenaline (epinephrine) (Primarily) | Chromaffin cells | Fight-or-flight response:
|
| Noradrenaline (norepinephrine) | Chromaffin cells | Fight-or-flight response:
|
| Dopamine | Chromaffin cells | Increase heart rate and blood pressure |
| Enkephalin | Chromaffin cells | Regulate pain |
Reproductive
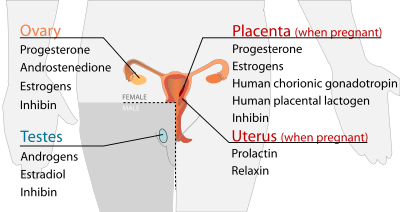
Testes
| Secreted hormone | From cells | Effect |
|---|---|---|
| Androgens (chiefly testosterone) | Leydig cells | Anabolic: growth of muscle mass and strength, increased bone density, growth and strength,
Virilizing: maturation of sex organs, formation of scrotum, deepening of voice, growth of beard and axillary hair. |
| Estradiol | Sertoli cells | Prevent apoptosis of germ cells[8] |
| Inhibin | Sertoli cells | Inhibit production of FSH |
Ovarian follicle and corpus luteum
| Secreted hormone | From cells | Effect |
|---|---|---|
| Progesterone | Granulosa cells, theca cells | Support pregnancy:[9]
Other:
|
| Androstenedione | Theca cells | Substrate for estrogen |
| Estrogens (mainly estradiol) | Granulosa cells | Structural:
Protein synthesis:
Fluid balance:
Gastrointestinal tract:
Melanin:
Cancer:
Lung function: |
| Inhibin | Granulosa cells | Inhibit production of FSH from anterior pituitary |
Placenta (when pregnant)
| Secreted hormone | Abbreviation | From cells | Effect |
|---|---|---|---|
| Progesterone (Primarily) | Support pregnancy:[9]
Other effects on mother similar to ovarian follicle-progesterone | ||
| Estrogens (mainly Estriol) (Also Primarily) | Effects on mother similar to ovarian follicle estrogen | ||
| Human chorionic gonadotropin | HCG | Syncytiotrophoblast | Promote maintenance of corpus luteum during beginning of pregnancy
Inhibit immune response, towards the human embryo. |
| Human placental lactogen | HPL | Syncytiotrophoblast | Increase production of insulin and IGF-1
Increase insulin resistance and carbohydrate intolerance |
| Inhibin | Fetal Trophoblasts | Suppress FSH |
Uterus (when pregnant)
| Secreted hormone | Abbreviation | From cells | Effect |
|---|---|---|---|
| Prolactin | PRL | Decidual cells | milk production in mammary glands |
| Relaxin | Decidual cells | Unclear in humans and animals |
Calcium regulation
Parathyroid
| Secreted hormone | Abbreviation | From cells | Effect |
|---|---|---|---|
| Parathyroid hormone | PTH | Parathyroid chief cell | Calcium:
|
Skin
| Secreted hormone | From cells | Effect |
|---|---|---|
| Calcidiol (25-hydroxyvitamin D3) | Inactive form of vitamin D3 |
Targets

Heart
| Secreted hormone | Abbreviation | From cells | Effect |
|---|---|---|---|
| Atrial-natriuretic peptide | ANP | Cardiac myocytes | Reduce blood pressure by:
reducing systemic vascular resistance, reducing blood water, sodium and fats |
| Brain natriuretic peptide | BNP | Cardiac myocytes | (To a lesser degree than ANP) reduce blood pressure by:
reducing systemic vascular resistance, reducing blood water, sodium and fats |
Bone marrow
| Secreted hormone | From cells | Effect |
|---|---|---|
| Thrombopoietin | liver and kidney cells | stimulates megakaryocytes to produce platelets[6] |
Skeletal muscle
In 1998, skeletal muscle was identified as an endocrine organ[14] due to its now well-established role in the secretion of myokines.[14][15] The use of the term myokine to describe cytokines and other peptides produced by muscle as signalling molecules was proposed in 2003.[16]
Adipose tissue
Signalling molecules released by adipose tissue are referred to as adipokines.
| Secreted hormone | From cells | Effect |
|---|---|---|
| Leptin (Primarily) | Adipocytes | decrease of appetite and increase of metabolism. |
| Estrogens[17] (mainly Estrone) | Adipocytes |
Major endocrine systems
The human endocrine system consists of several systems that operate via feedback loops. Several important feedback systems are mediated via the hypothalamus and pituitary.[18]
- TRH – TSH – T3/T4
- GnRH – LH/FSH – sex hormones
- CRH – ACTH – cortisol
- Renin – angiotensin – aldosterone
- leptin vs. insulin
Physiology
Interaction with immune system
Extensive bidirectional interactions exist between the endocrine system and the immune system.[19] Cortisol has major immunosuppressive effects,[20][21] and dopamine has immunomodulatory functions.[22] On the other hand, cytokines produced during inflammation activate the HPA axis at all three levels, sensible to negative feedback.[23] Moreover, cytokines stimulate hepcidin release from the liver, which is eventually responsible for the anemia of chronic disease.[24]
Other types of signalling
The typical mode of cell signaling in the endocrine system is endocrine signaling, that is, using the circulatory system to reach distant target organs. However, there are also other modes, i.e., paracrine, autocrine, and neuroendocrine signaling. Purely neurocrine signaling between neurons, on the other hand, belongs completely to the nervous system.
Autocrine
Autocrine signaling is a form of signaling in which a cell secretes a hormone or chemical messenger (called the autocrine agent) that binds to autocrine receptors on the same cell, leading to changes in the cells.
Paracrine
Some endocrinologists and clinicians include the paracrine system as part of the endocrine system, but there is not consensus. Paracrines are slower acting, targeting cells in the same tissue or organ. An example of this is somatostatin which is released by some pancreatic cells and targets other pancreatic cells.[1]
Juxtacrine
Juxtacrine signaling is a type of intercellular communication that is transmitted via oligosaccharide, lipid, or protein components of a cell membrane, and may affect either the emitting cell or the immediately adjacent cells.[25]
It occurs between adjacent cells that possess broad patches of closely opposed plasma membrane linked by transmembrane channels known as connexons. The gap between the cells can usually be between only 2 and 4 nm.[3]
Diseases

Diseases of the endocrine system are common,[27] including conditions such as diabetes mellitus, thyroid disease, and obesity. Endocrine disease is characterized by irregulated hormone release (a productive pituitary adenoma), inappropriate response to signaling (hypothyroidism), lack of a gland (diabetes mellitus type 1, diminished erythropoiesis in chronic renal failure), or structural enlargement in a critical site such as the thyroid (toxic multinodular goitre). Hypofunction of endocrine glands can occur as a result of loss of reserve, hyposecretion, agenesis, atrophy, or active destruction. Hyperfunction can occur as a result of hypersecretion, loss of suppression, hyperplastic or neoplastic change, or hyperstimulation.
Endocrinopathies are classified as primary, secondary, or tertiary. Primary endocrine disease inhibits the action of downstream glands. Secondary endocrine disease is indicative of a problem with the pituitary gland. Tertiary endocrine disease is associated with dysfunction of the hypothalamus and its releasing hormones.
As the thyroid, and hormones have been implicated in signaling distant tissues to proliferate, for example, the estrogen receptor has been shown to be involved in certain breast cancers. Endocrine, paracrine, and autocrine signaling have all been implicated in proliferation, one of the required steps of oncogenesis.[28]
Other common diseases that result from endocrine dysfunction include Addison’s disease, Cushing’s disease and Grave’s disease. Cushing's disease and Addison's disease are pathologies involving the dysfunction of the adrenal gland. Dysfunction in the adrenal gland could be due to primary or secondary factors and can result in hypercortisolism or hypocortisolism . Cushing’s disease is characterized by the hypersecretion of the adrenocorticotropic hormone (ACTH) due to a pituitary adenoma that ultimately causes endogenous hypercortisolism by stimulating the adrenal glands.[29] Some clinical signs of Cushing’s disease include obesity, moon face, and hirsutism.[2] Addison's disease is an endocrine disease that results from hypocortisolism caused by adrenal gland insufficiency. Adrenal insufficiency is significant because it is correlated with decreased ability to maintain blood pressure and blood sugar, a defect that can prove to be fatal.[30]
Graves' disease involves the hyperactivity of the thyroid gland which produces the T3 and T4 hormones.[2] Graves' disease effects range from excess sweating, fatigue, heat intolerance and high blood pressure to swelling of the eyes that causes redness, puffiness and in rare cases reduced or double vision.[3]
Other animals
A neuroendocrine system has been observed in all animals with a nervous system and all vertebrates have an hypothalamus-pituitary axis.[31] All vertebrates have a thyroid, which in amphibians is also crucial for transformation of larvae into adult form.[32][33] All vertebrates have adrenal gland tissue, with mammals unique in having it organized into layers.[34] All vertebrates have some form of renin-angiotensin axis, and all tetrapods have aldosterone as primary mineralocorticoid.[35][36]
Additional images
 Female endocrine system.
Female endocrine system. Male endocrine system
Male endocrine system
See also
References
- 1 2 3 Marieb, Elaine (2014). Anatomy & physiology. Glenview, IL: Pearson Education, Inc. ISBN 978-0321861580.
- 1 2 3 Vander, Arthur (2008). Vander's Human Physiology: the mechanisms of body function. Boston: McGraw-Hill Higher Education. pp. 345-347
- 1 2 3 Vander, Arthur (2008). Vander's Human Physiology: the mechanisms of body function. Boston: McGraw-Hill Higher Education. pp. 332–333.
- ↑ Colorado State University – Biomedical Hypertextbooks – Somatostatin
- ↑ Endo K, Matsumoto T, Kobayashi T, Kasuya Y, Kamata K (2005). "Diabetes-related changes in contractile responses of stomach fundus to endothelin-1 in streptozotocin-induced diabetic rats". J Smooth Muscle Res. 41 (1): 35–47. PMID 15855738. doi:10.1540/jsmr.41.35.
- 1 2 3 Kaushansky K (May 2006). "Lineage-specific hematopoietic growth factors". N Engl J Med. 354 (19): 2034–45. PMID 16687716. doi:10.1056/NEJMra052706.
- 1 2 Physiology: 5/5ch4/s5ch4_17 - Essentials of Human Physiology
- ↑ Pentikäinen V, Erkkilä K, Suomalainen L, Parvinen M, Dunkel L (2000). "Estradiol acts as a germinal cell survival factor in the human testis in vitro". J Clin Endocrinol Metab. 85 (5): 2057–67. PMID 10843196. doi:10.1210/jcem.85.5.6600.
- 1 2 3 4 Bowen, R. (August 6, 2000) Placental Hormones. Colorado State University
- ↑ Physiology: 5/5ch9/s5ch9_13 - Essentials of Human Physiology
- ↑ Hould F, Fried G, Fazekas A, Tremblay S, Mersereau W (1988). "Progesterone receptors regulate gallbladder motility". J Surg Res. 45 (6): 505–12. PMID 3184927. doi:10.1016/0022-4804(88)90137-0.
- ↑ Hormonal Therapy
- ↑ Massaro D, Massaro GD (2004). "Estrogen regulates pulmonary alveolar formation, loss, and regeneration in mice". American Journal of Physiology. Lung Cellular and Molecular Physiology. 287 (6): L1154–9. PMID 15298854. doi:10.1152/ajplung.00228.2004.
- 1 2 Pedersen BK, Febbraio MA (October 2008). "Muscle as an endocrine organ: focus on muscle-derived interleukin-6". Physiological Reviews. 88 (4): 1379–406. PMID 18923185. doi:10.1152/physrev.90100.2007.
- ↑ Ostrowski K, Hermann C, Bangash A, Schjerling P, Nielsen JN, Pedersen BK (December 1998). "A trauma-like elevation of plasma cytokines in humans in response to treadmill running". The Journal of Physiology. 513 (3): 889–94. PMC 2231318
 . PMID 9824725. doi:10.1111/j.1469-7793.1998.889ba.x.
. PMID 9824725. doi:10.1111/j.1469-7793.1998.889ba.x. - ↑ Pedersen BK, Steensberg A, Fischer C, et al. (2003). "Searching for the exercise factor: is IL-6 a candidate?". Journal of Muscle Research and Cell Motility. 24 (2–3): 113–9. PMID 14609022. doi:10.1023/A:1026070911202.
- ↑ Frühbeck G (July 2004). "The adipose tissue as a source of vasoactive factors". Curr Med Chem Cardiovasc Hematol Agents. 2 (3): 197–208. PMID 15320786. doi:10.2174/1568016043356255.
- ↑ Sherwood, L. (1997). "Human Physiology: From Cells to Systems". Wadsworth Pub Co. ISBN 0495391840.
- ↑ Turnbull AV, Rivier CL (January 1999). "Regulation of the hypothalamic-pituitary-adrenal axis by cytokines: actions and mechanisms of action". Physiological Reviews. 79 (1): 1–71. PMID 9922367.
- ↑ O'Connor TM, O'Halloran DJ, Shanahan F (June 2000). "The stress response and the hypothalamic-pituitary-adrenal axis: from molecule to melancholia" (PDF). QJM. 93 (6): 323–33. PMID 10873181. doi:10.1093/qjmed/93.6.323.
- ↑ Tsigos C, Chrousos GP (October 2002). "Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress". Journal of Psychosomatic Research. 53 (4): 865–71. PMID 12377295. doi:10.1016/S0022-3999(02)00429-4.
- ↑ Sarkar C, Basu B, Chakroborty D, Dasgupta PS, Basu S (May 2010). "The immunoregulatory role of dopamine: an update". Brain, Behavior, and Immunity. 24 (4): 525–8. PMC 2856781
 . PMID 19896530. doi:10.1016/j.bbi.2009.10.015.
. PMID 19896530. doi:10.1016/j.bbi.2009.10.015. - ↑ Silverman MN, Pearce BD, Biron CA, Miller AH (2005). "Immune modulation of the hypothalamic-pituitary-adrenal (HPA) axis during viral infection". Viral Immunology. 18 (1): 41–78. PMC 1224723
 . PMID 15802953. doi:10.1089/vim.2005.18.41.
. PMID 15802953. doi:10.1089/vim.2005.18.41. - ↑ Andrews NC (May 2004). "Anemia of inflammation: the cytokine-hepcidin link". The Journal of Clinical Investigation. 113 (9): 1251–3. PMC 398435
 . PMID 15124013. doi:10.1172/JCI21441.
. PMID 15124013. doi:10.1172/JCI21441. - ↑ Gilbert, Scott F. (2000-01-01). "Juxtacrine Signaling".
- ↑ "Mortality and Burden of Disease Estimates for WHO Member States in 2002" (xls). World Health Organization. 2002.
- ↑ Kasper (2005). Harrison's Principles of Internal Medicine. McGraw Hill. p. 2074. ISBN 0-07-139140-1.
- ↑ Bhowmick NA, Chytil A, Plieth D, Gorska AE, Dumont N, Shappell S, Washington MK, Neilson EG, Moses HL (2004). "TGF-beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia". Science. 303 (5659): 848–51. PMID 14764882. doi:10.1126/science.1090922.
- ↑ Buliman A, Tataranu LG, Paun DL, Mirica A, Dumitrache C (2016). "Cushing's disease: a multidisciplinary overview of the clinical features, diagnosis, and treatment". Journal Of Medicine & Life. 9 (1): 12–18.
- ↑ Inder, Warrick J.; Meyer, Caroline; Hunt, Penny J. (2015-06-01). "Management of hypertension and heart failure in patients with Addison's disease". Clinical Endocrinology. 82 (6): 789–792. ISSN 1365-2265. doi:10.1111/cen.12592.
- ↑ Hartenstein V (September 2006). "The neuroendocrine system of invertebrates: a developmental and evolutionary perspective". The Journal of Endocrinology. 190 (3): 555–70. PMID 17003257. doi:10.1677/joe.1.06964.
- ↑ Dickhoff, Walton W.; Darling, Douglas S. (1983). "Evolution of Thyroid Function and Its Control in Lower Vertebrates". American Zoologist. 23 (3): 697–707. JSTOR 3882951. doi:10.1093/icb/23.3.697.
- ↑ Galton, Valerie Anne (1 January 1988). "The Role of Thyroid Hormone in Amphibian Development". Integrative and Comparative Biology. 28 (2): 309–18. JSTOR 3883279. doi:10.1093/icb/28.2.309.
- ↑ Pohorecky LA, Wurtman RJ (March 1971). "Adrenocortical control of epinephrine synthesis". Pharmacological Reviews. 23 (1): 1–35. PMID 4941407.
- ↑ Wilson JX (1984). "The renin-angiotensin system in nonmammalian vertebrates". Endocrine Reviews. 5 (1): 45–61. PMID 6368215. doi:10.1210/edrv-5-1-45.
- ↑ Colombo L, Dalla Valle L, Fiore C, Armanini D, Belvedere P (April 2006). "Aldosterone and the conquest of land". Journal of Endocrinological Investigation. 29 (4): 373–9. PMID 16699307. doi:10.1007/bf03344112.
External links
| The Wikibook Human Physiology has a page on the topic of: The endocrine system |
| The Wikibook Anatomy and Physiology of Animals has a page on the topic of: Endocrine System |
| Library resources about Endocrine system |

