Estramustine phosphate
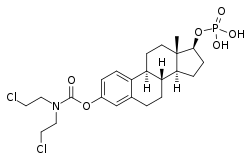 | |
| Clinical data | |
|---|---|
| Trade names | Emcyt, Estracyt[1] |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a608046 |
| License data |
|
| Pregnancy category | |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 75%[2] |
| Metabolism | Hepatic[2] |
| Metabolites | Estramustine, estromustine, normustine, estradiol, estrone, phosphoric acid[2] |
| Biological half-life | 15–24 hours[2] |
| Excretion | Feces (2.9–4.8%)[2] |
| Identifiers | |
| |
| Synonyms | Leo 299; NSC-89199; Ro 21-8837/001;[3] Estradiol 3-[bis(2-chloroethyl)carbamate] dihydrogen phosphate |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.019.161 |
| Chemical and physical data | |
| Formula | C23H32Cl2NO6P |
| Molar mass | 520.384 g/mol |
| 3D model (JSmol) | |
| |
| |
Estramustine phosphate, sold under the brand names Emcyt and Estracyt and provided as the sodium or meglumine salt, is a dual alkylating antineoplastic agent (i.e., a chemotherapy drug) of the nitrogen mustard type and hormonal antineoplastic agent of the estrogen type that is used in the treatment of prostate cancer.[3][1][4]
Medical uses
Estramustine phosphate is indicated, in the United States, for the palliative treatment of metastatic and/or progressive prostate cancer,[2] whereas in the United Kingdom it is indicated for the treatment of unresponsive or relapsing prostate cancer.[5][6][7][8]
Contraindications
Estramustine phosphate is contraindicated when used in children, patients hypersensitive to estrogen or nitrogen mustards, those with peptic ulcer (an ulcer in the digestive tract), those with severely compromised liver function, those with weak heart muscle (also known as myocardial insufficiency) and those with thromboembolic disorders or complications related to fluid retention.[5]
Side effects
Adverse effects by frequency:[2][5]
Very common (>10% frequency):
Common (1-10% frequency):
- Blood clots and complications thereof (including stroke, heart attack, pulmonary embolism, and thrombophlebitis)
Rare (<0.1% frequency):
- Angioedema, occurs most commonly when used in combination with ACE inhibitors.
Unlike other nitrogen mustards, estramustine phosphate seldom produces significant gastrointestinal or hematologic toxicity such as myelosuppression,[6] the major drug toxicity-related cause of drug discontinuation is thromboembolism (blood clots).[9]
Interactions
Estramustine phosphate have been reported to increase the toxicity and effectiveness of tricyclic antidepressants like amitriptyline and imipramine.[5] Dairy products like milk and other products containing calcium, aluminium, and magnesium like supplements have been reported to reduce the absorption of estramustine phosphate from the gastrointestinal tract hence reducing oral bioavailability.[5] There may be an increased risk of angioedema in those concurrently taking ACE inhibitors.[5]
Pharmacology
Pharmacodynamics
Estramustine phosphate acts by two mechanisms: 1) direct cytotoxic activity via inhibition of microtubules (caused by its alkylating nitrogen mustard moiety); and 2) antigonadotropic effects which suppress gonadal androgen production (caused by its estrogenic activity).[10] In regards to the latter, there is depolymerization of the microtubules (which estramustine phosphate achieves by binding to microtubule-associated proteins); this arrests prostate cancer cells in the G2/M phase of the cell cycle.[6][7][8] The drug is tissue-selectively taken up by prostate cells and hence produces minimal cytotoxic effects in healthy tissue.[6]
Pharmacokinetics
Estramustine phosphate is a prodrug and is delivered as an oral capsule. It is readily taken up from the gastrointestinal tract and then rapidly dephosphorylated into estramustine.[6][11] Estramustine is then partially oxidized to estromustine.[6] Some estramustine and estromustine undergoes hydrolysis at the ester bond in the liver to form the active metabolites normustine, estradiol, and estrone.[6][11]
| Estramustine | Estromustine | Estradiol | Estrone | Normustine |
|---|---|---|---|---|
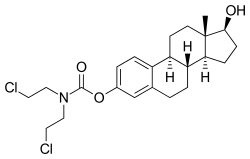 | 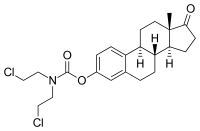 |  | 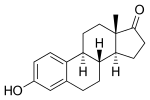 | 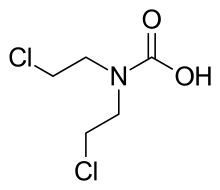 |
Chemistry
Estramustine phosphate is an estrane steroid and an estrogen ester. It is a derivative and diester of estradiol with a nitrogen mustard-carbamate ester moiety and a phosphate ester attached. Related although never-marketed drugs include alestramustine, atrimustine, cytestrol acetate, estradiol mustard, ICI-85966, and phenestrol.
Society and culture
Generic name
Estramustine phosphate is provided as the sodium salt estramustine phosphate sodium (USAN) or estramustine sodium phosphate (BANM, JAN) or as the meglumine salt estramustine phosphate meglumine, and these are the generic names of the drug.[1][12]
Availability
Estramustine phosphate is marketed in the United States,[13] Canada, and Mexico under the brand name Emcyt, whereas the drug is marketed under the brand name Estracyt in the United Kingdom and elsewhere throughout Europe as well as in Argentina, Chile, and Hong Kong.[1] It has been discontinued in a number of markets, including Australia, Brazil, Ireland, and Norway.[14]
See also
References
- 1 2 3 4 Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 406–407. ISBN 978-3-88763-075-1.
- 1 2 3 4 5 6 7 "Emcyt (estramustine) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. Retrieved 8 February 2014.
- 1 2 J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 502–503. ISBN 978-1-4757-2085-3.
- ↑ I.K. Morton; Judith M. Hall (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 114–. ISBN 978-94-011-4439-1.
- 1 2 3 4 5 6 "Estracyt Capsules - Summary of Product Characteristics (SPC)". electronic Medicines Compendium. Pfizer Limited. 12 August 2013. Retrieved 8 February 2014.
- 1 2 3 4 5 6 7 Perry, CM; McTavish, D (July 1995). "Estramustine Phosphate Sodium". Drugs & Aging. 7 (1): 49–74. PMID 7579781. doi:10.2165/00002512-199507010-00006.
- 1 2 Bergenheim, AT; Henriksson, R (February 1998). "Pharmacokinetics and pharmacodynamics of estramustine phosphate.". Clinical pharmacokinetics. 34 (2): 163–72. PMID 9515186. doi:10.2165/00003088-199834020-00004.
- 1 2 Simpson, D; Wagstaff, AJ (2003). "Estramustine Phosphate Sodium". American Journal of Cancer. 2 (5): 373–390. doi:10.2165/00024669-200302050-00013.
- ↑ Fizazi K, Le Maitre A, Hudes G, Berry WR, Kelly WK, Eymard JC, Logothetis CJ, Pignon JP, Michiels S (2007). "Addition of estramustine to chemotherapy and survival of patients with castration-refractory prostate cancer: a meta-analysis of individual patient data". Lancet Oncol. 8 (11): 994–1000. PMID 17942366. doi:10.1016/S1470-2045(07)70284-X.
- ↑ Michael Oettel; Ekkehard Schillinger (6 December 2012). Estrogens and Antiestrogens II: Pharmacology and Clinical Application of Estrogens and Antiestrogen. Springer Science & Business Media. pp. 540–. ISBN 978-3-642-60107-1.
- 1 2 Franco Cavalli; Stan B. Kaye`; Heine H Hansen; James O Armitage; Martine Piccart-Gebhart (12 September 2009). Textbook of Medical Oncology, Fourth Edition. CRC Press. pp. 442–. ISBN 978-0-203-09289-7.
- ↑ https://www.drugs.com/international/estramustine.html
- ↑ "Drugs@FDA: FDA Approved Drug Products". United States Food and Drug Administration. Retrieved 29 January 2017.
- ↑ Sweetman, S, ed. (12 February 2013). "Estramustine Sodium Phosphate". Martindale: The Complete Drug Reference. London, UK: Pharmaceutical Press. Retrieved 8 February 2014.