Embryology

Embryology (from Greek ἔμβρυον, embryon, "the unborn, embryo"; and -λογία, -logia) is the branch of biology that studies the prenatal development of gametes (sex cells), fertilization, and development of embryos and fetuses. Additionally, embryology encompasses the study of congenital disorders that occur before birth, known as teratology.[1]
Embryonic development of animals
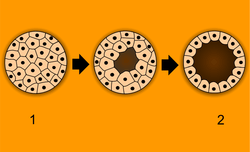
After cleavage, the dividing cells, or morula, becomes a hollow ball, or blastula, which develops a hole or pore at one end.
Bilateria
In bilateral animals, the blastula develops in one of two ways that divides the whole animal kingdom into two halves (see: Embryological origins of the mouth and anus). If in the blastula the first pore (blastopore) becomes the mouth of the animal, it is a protostome; if the first pore becomes the anus then it is a deuterostome. The protostomes include most invertebrate animals, such as insects, worms and molluscs, while the deuterostomes include the vertebrates. In due course, the blastula changes into a more differentiated structure called the gastrula.
The gastrula with its blastopore soon develops three distinct layers of cells (the germ layers) from which all the bodily organs and tissues then develop:
- The innermost layer, or endoderm, gives a rise to the digestive organs, the gills, lungs or swim bladder if present, and kidneys or nephrites.
- The middle layer, or mesoderm, gives rise to the muscles, skeleton if any, and blood system.
- The outer layer of cells, or ectoderm, gives rise to the nervous system, including the brain, and skin or carapace and hair, bristles, or scales.
Embryos in many species often appear similar to one another in early developmental stages. The reason for this similarity is because species have a shared evolutionary history. These similarities among species are called homologous structures, which are structures that have the same or similar function and mechanism, having evolved from a common ancestor.
Drosophila melanogaster (fruit fly)


Drosophila melanogaster, a fruit fly, is a model organism in biology on which much research into embryology has been done.[2] Before fertilization, the female gamete produces an abundance of mRNA - transcribed from the genes that encode bicoid protein and nanos protein.[3][4] These mRNA molecules are stored to be used later in what will become the developing embryo. The male and female Drosophila gametes exhibit anisogamy (differences in morphology and sub-cellular biochemistry). The female gamete is larger than the male gamete because it harbors more cytoplasm and, within the cytoplasm, the female gamete contains an abundance of the mRNA previously mentioned.[5][6] At fertilization, the male and female gametes fuse (plasmogamy) and then the nucleus of the male gamete fuses with the nucleus of the female gamete (karyogamy). Note that before the gametes' nuclei fuse, they are known as pronuclei.[7] A series of nuclear divisions will occur without cytokinesis (division of the cell) in the zygote to form a multi-nucleated cell (a cell containing multiple nuclei) known as a syncytium.[8][9] All the nuclei in the syncytium are identical, just as all the nuclei in every somatic cell of any multicellular organism are identical in terms of the DNA sequence of the genome.[10] Before the nuclei can differentiate in transcriptional activity, the embryo (syncytium) must be divided into segments. In each segment, a unique set of regulatory proteins will cause specific genes in the nuclei to be transcribed. The resulting combination of proteins will transform clusters of cells into early embryo tissues that will each develop into multiple fetal and adult tissues later in development (note: this happens after each nucleus becomes wrapped with its own cell membrane).
Outlined below is the process that leads to cell and tissue differentiation.
Maternal-effect genes - subject to Maternal (cytoplasmic) inheritance.
- Egg-polarity genes establish the Anteroposterior axis.[11]
Zygotic-effect genes - subject to Mendelian (classical) inheritance.
- Segmentation genes establish 14 segments of the embryo using the anteroposterior axis as a guide.[12][13]
- Gap genes establish 3 broad segments of the embryo.[14][15][16]
- Pair-rule genes define 7 segments of the embryo within the confines of the second broad segment that was defined by the gap genes.[17]
- Segment-polarity genes define another 7 segments by dividing each of the pre-existing 7 segments into anterior and posterior halves.[18][19]
- Homeotic (homeobox) genes use the 14 segments as pinpoints for specific types of cell differentiation and the histological developments that correspond to each cell type.[20][21]
Humans
Humans are bilaterals and deuterostomes.
In humans, the term embryo refers to the ball of dividing cells from the moment the zygote implants itself in the uterus wall until the end of the eighth week after conception. Beyond the eighth week after conception (tenth week of pregnancy), the developing human is then called a fetus.
History

As recently as the 18th century, the prevailing notion in western human embryology was preformation: the idea that semen contains an embryo – a preformed, miniature infant, or homunculus – that simply becomes larger during development. The competing explanation of embryonic development was epigenesis, originally proposed 2,000 years earlier by Aristotle. Much early embryology came from the work of the Italian anatomists Aldrovandi, Aranzio, Leonardo da Vinci, Marcello Malpighi, Gabriele Falloppio, Girolamo Cardano, Emilio Parisano, Fortunio Liceti, Stefano Lorenzini, Spallanzani, Enrico Sertoli, and Mauro Rusconi.[22] According to epigenesis, the form of an animal emerges gradually from a relatively formless egg. As microscopy improved during the 19th century, biologists could see that embryos took shape in a series of progressive steps, and epigenesis displaced preformation as the favoured explanation among embryologists.[23]
After 1827 and Before 1950
Karl Ernst von Baer and Heinz Christian Pander proposed the germ layer theory of development; von Baer discovered the mammalian ovum in 1827.[24][25][26] Modern embryological pioneers include Charles Darwin, Ernst Haeckel, J.B.S. Haldane, and Joseph Needham. Other important contributors include William Harvey, Kaspar Friedrich Wolff, Heinz Christian Pander, August Weismann, Gavin de Beer, Ernest Everett Just, and Edward B. Lewis.
After 1950
After the 1950s, with the DNA helical structure being unravelled and the increasing knowledge in the field of molecular biology, developmental biology emerged as a field of study which attempts to correlate the genes with morphological change, and so tries to determine which genes are responsible for each morphological change that takes place in an embryo, and how these genes are regulated.
 A study of embryos by Leonardo da Vinci.
A study of embryos by Leonardo da Vinci.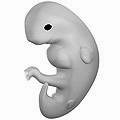 Human embryo at six weeks gestational age.
Human embryo at six weeks gestational age.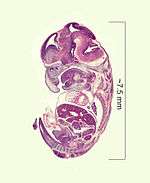 Histological film 10-day mouse embryo.
Histological film 10-day mouse embryo.
Vertebrate and invertebrate embryology
Many principles of embryology apply to invertebrates as well as to vertebrates.[27] Therefore, the study of invertebrate embryology has advanced the study of vertebrate embryology. However, there are many differences as well. For example, numerous invertebrate species release a larva before development is complete; at the end of the larval period, an animal for the first time comes to resemble an adult similar to its parent or parents. Although invertebrate embryology is similar in some ways for different invertebrate animals, there are also countless variations. For instance, while spiders proceed directly from egg to adult form, many insects develop through at least one larval stage.
Modern embryology research
Currently, embryology has become an important research area for studying the genetic control of the development process (e.g. morphogens), its link to cell signalling, its importance for the study of certain diseases and mutations, and in links to stem cell research.
See also
- Abortion
- Cell signalling
- Deuterostomes
- Developmental biology
- Embryo drawing
- Embryogenesis
- Embryology of digestive system and the body cavities
- Epigenesis (biology)
- French flag model
- Germ layers
- Hedgehog signaling pathway
- Hox gene
- Morphogens
- Ontogeny
- Plant embryogenesis, the development of a plant embryo from a fertilized ovum
- Plant physiology
- Prenatal development
- Protostomes
- Recapitulation theory
References
Citations
- ↑ "Embryology Definition".
- ↑ Carlson, Bruce M.; Kantaputra, Piranit N. (2014). "4 Molecular Basis for Embryonic Development". Human embryology and developmental biology (5th ed.). Philadelphia, PA: Elsevier/Saunders. p. 59. ISBN 978-1-4557-2794-0.
the basic framework for understanding the molecular basis of embryonic development still rests largely on studies of developmental genetics in Drosophila
- ↑ Alberts, Bruce; Johnson, Alexander; Lewis, Julian; Raff, Martin; Roberts, Keith; Walter, Peter (2008). "22 Development of Multicellular Organisms". Molecular biology of the cell (5th ed.). New York: Garland Science. p. 1334. ISBN 0-8153-4106-7.
All the egg-polarity genes in these four classes are maternal-effect genes: it is the mother’s genome, not the zygotic genome, that is critical. Thus, a fly whose chromosomes are mutant in both copies of the Bicoid gene but who is born from a mother carrying one normal copy of Bicoid develops perfectly normally, with- out any defects in the head pattern. However, if that daughter fly is a female no functional Bicoid mRNA can be deposited into the anterior part of her own eggs, and all of these will develop into headless embryos regardless of the father’s genotype.
- ↑ Alberts, Bruce; Johnson, Alexander; Lewis, Julian; Raff, Martin; Roberts, Keith; Walter, Peter (2008). "22 Development of Multicellular Organisms". Molecular biology of the cell (5th ed.). New York: Garland Science. p. 1334. ISBN 0-8153-4106-7.
The future posterior end of the embryo con- tains a high concentration of mRNA for a regulator of translation called Nanos, which sets up a posterior gradient in the same way. The third signal is generated symmetrically at both ends of the egg
- ↑ Farley, Brian M.; Ryder, Sean P. (January 2008). "Regulation of Maternal mRNAs in Early Development". Critical Reviews in Biochemistry and Molecular Biology. 43 (2): 135–162. doi:10.1080/10409230801921338.
Most sexually reproducing metazoans are anisogamous, meaning that the two gametes that combine during fertilization differ greatly in size. By convention, the larger gametes are considered female and are called ova, while the smaller gametes are male and are called sperm. In most cases, both gametes contribute similarly to the chromosomal content of the new organism. In contrast, the maternal gamete contributes nearly all of the cytoplasm. This cytoplasmic contribution is crucial to patterning early development; it contains the maternal proteins and transcripts that guide the early steps of development prior to the activation of zygotic transcription.
- ↑ Driever, Wolfgang; Nüsslein-Volhard, Christiane (July 1988). "A gradient of bicoid protein in Drosophila embryos". Cell. 54 (1): 83–93. doi:10.1016/0092-8674(88)90182-1.
The maternal gene Bicoid (BCD) organizes anterior development in Drosophila. Its mRNA is localized at the anterior tip of the oocyte and early embryo. Antibodies raised against bcd fusion proteins recognize a 55–57 kd doublet band in Western blots of extracts of 0–4 hr old embryos. This protein is absent or reduced in embryonic extracts of nine of the 11 bcd alleles. The protein is concentrated in the nuclei of cleavage stage embryos. It cannot be detected in oocytes, indicating temporal control of bcd mRNA translation. The bcd protein is distributed in an exponential concentration gradient with a maximum at the anterior tip, reaching background levels in the posterior third of the embryo. The gradient is probably generated by diffusion from the local mRNA source and dispersed degradation.
- ↑ Carlson, Bruce M.; Kantaputra, Piranit N. (2014). "2 Transport of Gametes and Fertilization". Human embryology and developmental biology (5th ed.). Philadelphia, PA: Elsevier/Saunders. p. 59. ISBN 978-1-4557-2794-0.
after the head of the sperm enters the cytoplasm of the egg... the chromatin begins to spread out within the nucleus (now called a pronucleus) as it moves closer to the nuclear material of the egg.
- ↑ Warn, RM (1986). "The cytoskeleton of the early Drosophila embryo.". Journal of cell science. Supplement. 5: 311–28. PMID 3308915.
This type of embryo shows a separation of mitosis from cytokinesis during the early stages of development. Most cells are only formed when a syncytium of approximately 6000 nuclei are present.
- ↑ Safir, Shelley R. (1920). "Genetic and Cytological Examination of the Phenomena of Primary Non-Disjunction in Drosophila melanogaster". Genetics. 5: 459–87. PMC 1200490
 . PMID 17245950.
. PMID 17245950. A rounded syncytium was thus formed that contained two, four, or eight nuclei
- ↑ Wilmut, I.; Schnieke, A. E.; McWhir, J.; Kind, A. J.; Campbell, K. H. S. (27 February 1997). "Viable offspring derived from fetal and adult mammalian cells". Nature. 385 (6619): 810–813. PMID 9039911. doi:10.1038/385810a0.
Transfer of a single nucleus at a specific stage of development, to an enucleated unfertilized egg, provided an opportunity to investigate whether cellular differentiation to that stage involved irreversible genetic modification... The fact that a lamb was derived from an adult cell confirms that differentiation of that cell did not involve the irreversible modification of genetic material required for development to term
- ↑ Alberts, Bruce; Johnson, Alexander; Lewis, Julian; Raff, Martin; Roberts, Keith; Walter, Peter (2008). "22 Development of Multicellular Organisms". Molecular biology of the cell (5th ed.). New York: Garland Science. pp. 1333–1334. ISBN 0-8153-4106-7.
In the stages before fertilization, the anteroposterior axis of the future embryo becomes defined by three systems of molecules that create landmarks in the oocyte (Figure 22–32)... The three sets of genes responsible for these localized deter- minants are referred to as the anterior, posterior, and terminal sets of egg- polarity genes.
- ↑ Nusslein-Volhard, C.; Kluding, H.; Jurgens, G. (1 January 1985). "Genes Affecting the Segmental Subdivision of the Drosophila Embryo". Cold Spring Harbor Symposia on Quantitative Biology. 50 (0): 145–154. doi:10.1101/SQB.1985.050.01.020.
In Drosophila, a number of genes have been identified that are involved in segmentation
- ↑ Alberts, Bruce; Johnson, Alexander; Lewis, Julian; Raff, Martin; Roberts, Keith; Walter, Peter (2008). "22 Development of Multicellular Organisms". Molecular biology of the cell (5th ed.). New York: Garland Science. p. 1336. ISBN 0-8153-4106-7.
After the initial gradients of Bicoid and Nanos are created to define the antero- posterior axis, the segmentation genes refine the pattern.
- ↑ Jäckle, Herbert; Hoch, Michael; Pankratz, Michael J.; Gerwin, Nicole; Sauer, Frank; Brönner, Günter (January 1992). "Transcriptional control by Drosophila gap genes". Journal of Cell Science: 39–51.
The segmented body pattern along the longitudinal axis of the Drosophila embryo is established by a cascade of specific transcription factor activities. This cascade is initiated by maternal gene products that are localized at the polar regions of the egg. The initial long-range positional information of the maternal factors, which are transcription factors (or are factors which activate or localize transcription factors), is transferred through the activity of the zygotic segmentation genes. The gap genes act at the top of this regulatory hierarchy. Expression of the gap genes occurs in discrete domains along the longitudinal axis of the preblastoderm and defines specific, overlapping sets of segment primordia.
- ↑ Nüsslein-Volhard, C; Wieschaus, E (30 October 1980). "Mutations affecting segment number and polarity in Drosophila.". Nature. 287 (5785): 795–801. PMID 6776413. doi:10.1038/287795a0.
The phenotypes of the mutant embryos indicate that the process of segmentation involves at least three levels of spatial organization
- ↑ Jäckle, Herbert; Tautz, Diethard; Schuh, Reinhard; Seifert, Eveline; Lehmann, Ruth (18 December 1986). "Cross-regulatory interactions among the gap genes of Drosophila". Nature. 324 (6098): 668–670. doi:10.1038/324668a0.
Zygotic expression of the gap genes is thought to be required for the subdivision of the embryo into several units of adjacent segments
- ↑ Harding, K; Rushlow, C; Doyle, H.; Hoey, T; Levine, M (29 August 1986). "Cross-regulatory interactions among pair-rule genes in Drosophila". Science. 233 (4767): 953–959. doi:10.1126/science.3755551.
The pair-rule genes of Drosophila are required for the subdivision of the developing embryo into a repeating series of homologous body segments. One of the pair-rule genes, even-skipped (eve), appears to be particularly important for the overall segmentation pattern since eve- embryos lack all segmental subdivisions in the middle body region.
- ↑ Martizez Arias, A; Baker, NE; Ingham, PW (May 1988). "Role of segment polarity genes in the definition and maintenance of cell states in the Drosophila embryo.". Development (Cambridge, England). 103 (1): 157–70. PMID 3197626.
Segment polarity genes are expressed and required in restricted domains within each metameric unit of the Drosophila embryo.
- ↑ Carlson, Bruce M.; Kantaputra, Piranit N. (2014). "4 Molecular Basis for Embryonic Development". Human embryology and developmental biology (5th ed.). Philadelphia, PA: Elsevier/Saunders. p. 59. ISBN 978-1-4557-2794-0.
In Drosophila, each stripe (segment) is subdivided into anterior and posterior halves. The posterior half of one segment and the anterior half of the next are collectively known as a parasegment.
- ↑ Laughon, A.; Carroll, S.B.; Storfer, F.A.; Riley, P.D.; Scott, M.P. (1 January 1985). "Common Properties of Proteins Encoded by the Antennapedia Complex Genes of Drosophila melanogaster". Cold Spring Harbor Symposia on Quantitative Biology. 50 (0): 253–262. doi:10.1101/SQB.1985.050.01.032.
Evidence available to date, primarily from the study of Drosophila melanogaster, indicates that homeotic genes act to control development by selecting among alternative developmental pathways or programs. Many of the homeotic genes of Drosophila are clustered in two complexes called the bithorax complex (BX-C) (Lewis 1952, 1963, 1978, 1982) and the Antennapedia complex (ANT-C) (Kaufman et al. 1980). Genes of the BX-C regulate pattern formation in part of the thorax and in the abdomen, whereas different genes of the ANT-C function in the head, thorax, and abdomen. Genes in both complexes are commonly described as regulating “segmental identity.”
- ↑ Gehring, Walter J. (January 1985). "The homeo box: A key to the understanding of development?". Cell. 40 (1): 3–5. doi:10.1016/0092-8674(85)90300-9.
Development is based on the differential expression of the genetic information in a precise spatial and temporal pattern. The interdependence of various parts of a developing organism essentially excludes the possibility that each structural gene is regulated independently, but rather suggests some form of hierarchical control in which the controlling genes interact to regulate the activity of groups of structural genes in a concerted fashion. The recent isolation of homeotic genes and the discovery of the homeo box appear to be decisive steps toward the identification of these developmental controlling genes.
- ↑ De Felici Massimo; Siracus Gregorio (2000). "The rise of embryology in Italy: from the Renaissance to the early 20th Century" (PDF). Int. J. Dev. Biol. 44: 515–521.
- ↑ Campbell et al. (p. 987)
- ↑ K. J. Betteridge (1981). "An historical look at embryo transfer" (PDF). Reproduction. the Journal of the Society for Reproduction and Fertility. 62 (1): 1–13. doi:10.1530/jrf.0.0620001.
Three years later, the Estonian, Karl Ernst von Baer, finally found the true mammalian egg in a pet dog (von Baer, 1827).
- ↑ Lois N. Magner (2005). History of the Life Sciences. New York. Basel: Marcel Dekker. p. 166. ISBN 9780824743604.
- ↑ Alex Lopata (2009). "History of the Egg in Embryology". Journal of Mammalian Ova Research. 26: 2–9. doi:10.1274/jmor.26.2.
- ↑ Parker, Sybil. "Invertebrate Embryology," McGraw-Hill Encyclopedia of Science & Technology (McGraw-Hill 1997).
Sources
- Embryology - History of embryology as a science." Science Encyclopedia. Web. 06 Nov. 2009. <http://science.jrank.org/pages/2452/Embryology.html>.
- "Germ layer." Encyclopædia Britannica. 2009. Encyclopædia Britannica Online. 06 Nov. 2009 <http://www.britannica.com/EBchecked/topic/230597/germ-layer>.
Further reading
- Apostoli, Pietro; Catalani, Simona (2011). "Chapter 11. Metal Ions Affecting Reproduction and Development". In Astrid Sigel, Helmut Sigel and Roland K. O. Sigel. Metal Ions in Toxicology. Metal Ions in Life Sciences. 8. RSC Publishing. pp. 263–303. doi:10.1039/9781849732116-00263.
- Scott F. Gilbert. Developmental Biology. Sinauer, 2003. ISBN 0-87893-258-5.
- Lewis Wolpert. Principles of Development. Oxford University Press, 2006. ISBN 0-19-927536-X.
- Carlson, Bruce M.; Kantaputra, Piranit N. (2014). Human embryology and developmental biology. Philadelphia, PA: Elsevier/Saunders. ISBN 978-1-4557-2794-0. (click here for more information)
External links
| Wikimedia Commons has media related to Embryology. |
| Wikisource has the text of the 1911 Encyclopædia Britannica article Embryology. |
- Online course in embryology
- Indiana University's Human Embryology Animations
- UNSW Embryology Large resource of information and media.
- What is a human admixed embryo?
- Definition of embryo according to Webster
- Embryonews _ resource for IVF and embryology articles