Effects of alcohol on memory
Ethanol is the type of alcohol found in alcoholic beverages. It is a volatile, flammable, colorless liquid that acts as a central nervous system depressant.[1] Ethanol can impair different types of memory.
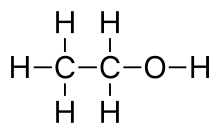

Mode of action
Effects on hippocampus
Alcohol acts as a general central nervous system depressant, but it also affects specific areas of the brain to a greater extent than others. Memory impairment due to alcohol has been linked to disruption of hippocampal function—in particular affecting gamma-Aminobutyric acid (GABA) and N-methyl-D-aspartate (NMDA) neurotransmission which negatively impacts long-term potentiation (LTP).[2] The molecular basis of LTP is associated with learning and memory.[3] Particularly, damage to hippocampal CA1 cells adversely affects memory formation,[4] and this disruption has been linked to dose-dependent levels of alcohol consumption.[5] At higher doses, alcohol significantly inhibits neuronal activity in the CA1 and CA3 pyramidal cell layers of the hippocampus.[6][7][8] This impairs memory encoding[9] since the hippocampus plays an important role in the formation of new memories.
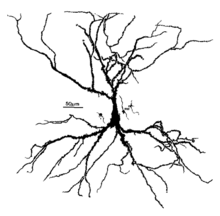
Molecular effects on GABA and NMDA receptors

Alcohol acts as a positive allosteric modulator of GABA receptors, specifically type GABAA.[10] Upon activation, these GABA receptors conduct Cl-, resulting in neuronal hyperpolarization. This hyperpolarization decreases the chance of an action potential occurring and, thus, has an inhibitory effect on neurotransmission in the central nervous system. GABAA receptor subtypes vary in their sensitivity to dosage of alcohol consumed. Furthermore, acute alcohol intake promotes GABAergic neurotransmission via presynaptic release of GABA, dephosphorylation of GABAA receptors (increasing GABA sensitivity), and elevation of endogenous GABAergic neuroactive steroids.[11] Protein kinase C (PKC) has been implicated in differentially modulating the response of the GABAA receptor to alcohol, with effects depending on the PKC isozyme.[12] Alcohol effects have also implicated protein kinase A in affecting GABAA receptor function, such as promoting sensitivity.[13] Enhancement of GABAergic transmission due to alcohol consumption can also be brought about by neuroactive steroids, such as allopregnanolone, which act as GABAA receptor agonists.[11][14] Chronic alcohol consumption and alcohol dependence are correlated with altered expression, properties, and functions of the GABAA receptor that may contribute to alcohol tolerance.[11] There is still much to be discovered about alcohol’s specific and varying effects on the GABAA receptor and its subtypes.
At higher doses, ethanol also affects NMDA receptors (NMDARs) by inhibiting the ion current induced by NMDA, a glutamate receptor agonist.[15] This inhibition of synaptic excitation by alcohol has been shown to be dose-dependent (up to a certain point, after which it did not differ by much).[16] Alcohol appears to produce this inhibition by using a site of the NMDAR that is accessible from the extracellular environment.[17] Thusly, this inhibition of an ion current usually produced by NMDAR activation leads to decreased LTP in hippocampal areas.[18] Alcohol negatively affects LTP to a greater degree in immature versus mature animals.[19] In adolescents, alcohol decreases the expression of the NMDAR NR2A subunit in the hippocampus and the NR1 subunit in the prefrontal cortex.[20] Studies have also found a decrease in phosphorylation of 2B subunit in the prefrontal cortex, hippocampus, nucleus accumbens, and striatum.[21] NMDARS may be affected by PKA regulation due to the actions of alcohol.[22] Alcohol’s effects on GABAA neurotransmission may indirectly inhibit the activity of the NMDAR and contribute to its blockade of LTP induction; however, alcohol’s direct effects on NMDAR alone are sufficient for the inhibition of LTP.[23] The varying dose-dependent response to alcohol relies on the combined interactions and responses of the GABAA receptors, NMDARs, and metabotropic glutamate receptors subtype 5 (mGluR5).[24][25][26] These changes prevent excitatory synaptic transmissions from occurring, affecting synaptic plasticity and, in turn, memory and learning. However, there is still much to be elucidated concerning specific molecular mechanisms of how alcohol affects memory formation.
Effects on other brain regions
Alcohol also impairs and alters functioning in the cerebellum, which affects motor function and coordination.[27] It has a notable inhibitory effect on neurons of the cerebral cortex, affecting and altering thought processes, decreasing inhibition, and increasing the pain threshold. It also decreases sexual performance by depressing nerve centers in the hypothalamus.[28][29] Alcohol also has an effect on urine excretion via inhibition of anti-diuretic hormone (ADH) secretion of the pituitary gland. Lastly, it depresses breathing and heart rate by inhibiting neuronal functioning of the medulla.[30]
Long-term memory
Long-term memory (LTM) has a long duration and large capacity.[31] Memories stored in LTM can last from a few days to a lifetime.[31] LTM consists of explicit memory (requiring conscious awareness) and implicit memory (unconscious awareness).[31] Information selected for LTM goes through three processes. First, in the encoding stage, information from the senses is incorporated into mental activity in the form of a memory.[31] Secondly, storage involves taking this information and holding it indefinitely in memory.[31] Lastly, retrieval is the ability to recall information from the long-term memory storage. Each of these processes can be affected by alcohol.[31]
Explicit memory
Explicit memory requires conscious and intentional effort for recall.[31] It includes both episodic memory (for specific events, such as a party) and semantic memory (for general information, such as one's name).[31]
Alcohol impairs episodic encoding, specifically for cued recall, recognition of completed word fragments, and free recall.[32] A blackout is an example of a difficulty in encoding episodic memories due to alcohol. Blackouts are caused by a rapid increase in blood alcohol concentration (BAC) which in turn distorts the neurons in the hippocampus.[33] This distortion impairs a person's ability to form new episodic memories.[33]
High doses of alcohol severely disrupt the storage process of semantic memories.[34] Alcohol was found to impair the storage of novel stimuli but not that of previously learned information.[34] Since alcohol affects the central nervous system, it hinders semantic storage functioning by restricting the consolidation of the information from encoding.[34]
Retrieval of explicit memory is significantly impaired by alcohol. When compared to sober participants, intoxicated participants performed quite poorly on a recall task for everyday events (episodic memory).[35] Intoxicated participants are also slower to respond in reaction time tasks.[36] Alcohol also impairs retrieval in word recognition tasks.[32] When both encoding and retrieval take place during intoxication, there are surprisingly more impairments for cued recall than for free recall.[32] In terms of gender differences in retrieval processes, females tend to score lower than males on recall tasks when intoxicated.[36]
Implicit memory
Implicit memory does not require conscious effort or intention for recall.[31] It occurs when previous experience influences performance on a certain task.[31] This is evident in priming experiments. Implicit memory includes procedural memory, which influences our everyday behaviours, such as riding a bike or tying shoes.[31] People can perform these abilities without thinking about them, which means procedural memory functions automatically. While retrieval of explicit memory is severely impaired by alcohol, retrieval of implicit memory is not.[35] Intoxicated subjects score higher on recognition tasks (involving implicit memory) than on recall tasks (involving explicit memory).[35]
Short-term memory
Short-term memory refers to temporary storage of small amounts of information over short delays.[31] Digit span refers to the proposed number of pieces of information (5-9) that can be held in short-term memory. This is also referred to as the magic number seven – plus or minus two. Any more pieces of information than this, and newer items replace previous items.[37] Alcohol intoxication has been found to have dissociative effects on short-term memory and cognitive functioning.[38][39]


Brain areas affected by alcohol
Alcohol affects brain functioning. Neurochemical changes occurring in the anterior cingulate are correlated with altered short-term memory functions in the brains of young alcoholic men.[40] fMRIs of alcohol-dependent women displayed significantly less blood oxygen in the frontal and parietal regions, especially in the right hemisphere.[41] This is supported by findings of short-term memory impairment by lesions of the parietal lobe[42] and prefrontal cortex.[43] Associations between Third ventricle volume and cognitive performance on memory tests have been found in alcoholics.[44] Specifically, increases in third ventricular volume correlate with a decline in memory performance.[44]
Tasks and intoxication findings
Short-term memory is commonly tested with visual tasks. Short-term memory, especially for non-verbal and spatial material, are impaired by intoxication.[44] Alcohol decreases iconic memory (a type of visual short-term memory).[45] With BACs between 80–84 mg/dl, more intrusion errors occur in a delayed recall task compared to a control group.[46] Intrusion errors, which represent reflective cognitive functioning, occur when irrelevant information is produced. Alcoholics have less control of inhibiting intrusions.[46] Acute alcohol intoxication in social drinkers caused more intrusion errors in delayed recall tasks than in immediate free recall tasks.[46] Acute alcohol intoxication increases susceptibility to interference, which allows for more intrusion errors when there is a short delay.[46] Free recall (given list of words then asked to recall list) is significantly lower and therefore impaired by alcohol intoxication.[47][48] Encoding deficits were found in verbal free recall and recognition tasks under the influence of alcohol.[49] A discrimination task found significant alcohol-related impairments in depth perception and in visual short-term memory.[50] State-dependent learning and relearning studies in male heavy drinkers demonstrate that the condition of intoxication while learning and sobriety when tested caused a performance deficit in free recall tasks.[51] These findings are supportive of alcohol-induced storage deficits (not retrieval deficits).[51] The effects of acute alcohol consumption on visual short-term memory, stereoscopic depth perception, and attention were studied. A 33% alcohol condition showed significant impairments in depth perception and in visual short-term memory (assessed by the vernier discrimination task).[52]

Effects on working memory
Working memory allows one to keep things in mind while simultaneously performing complex tasks.[31] It involves a system for the temporary storage and manipulation of information, forming a crucial link between perception and controlled action.[53] Evidence suggests that working memory involves three components: the central executive which controls attention, the visuo-spatial sketchpad which holds and manipulates spatial information, and the phonological loop which performs a similar function for auditory and speech-based information.[53]
In the short term
Alcohol consumption has substantial, measurable effects on working memory, though these effects vary greatly between individual responses. Little is known about the neural mechanisms that underlie these individual differences.[54] It is also found that alcohol impairs working memory by affecting mnemonic strategies and executive processes rather than by shrinking the basic holding capacity of working memory. Isolated acute-moderate levels of alcohol intoxication do not profoundly physically alter the structures which are critical for working memory function, such as the frontal cortex, parietal cortex, anterior cingulate, and parts of the basal ganglia.[38] One finding regarding the effects of alcohol on working memory points out that alcohol reduces working memory only in individuals with a high baseline working memory capacity,[55] which suggests that alcohol might not uniformly affect working memory in different individuals. Alcohol appears to impair the capacity of working memory to modulate response inhibition.[55] Alcohol disinhibits behaviour, but only in individuals with a low baseline working memory capacity.[55] An interesting finding is that incentive to perform well with working memory measurement tasks while under the influence of alcohol 'does in fact have some effect on working memory, in that it boosts scores in rate of mental scanning and reaction time to stimulus, but did not reduce number of errors compared to subjects with no incentive to perform well.[56] Even acute alcohol intoxication (a blood alcohol concentration of 0.08-0.09%) produces a substantial impairment of working memory processes that require mnemonic rehearsal strategies.[38] Alcohol is less likely to impair a working memory task that does not rely on memory rehearsal or associated mnemonic strategies.[38] Because of this, working memory is very susceptible to falter when a person is participating in tasks involving retention concerning auditory and visual sequences.[38] A real world and interesting example of this is failure of guitarists or other musicians performing concerts to cue in on auditory patterns and make it known that their performance is hindered by intoxication, whereas professional basketball (a less sequence-heavy activity for working memory) standout Ron Artest recently admitted in an interview with Sporting News to drinking heavily during half-time in the early days of his career and it having little if any recognizable effect on his working memory. His former coach Fran Fraschilla has gone on record saying:
"It's a surprise because every day at practice, he came out in a mood to play. He came out in a basketball rage. He was fully committed; he wanted to let our upperclassmen know that he was the alpha male. It never came up that he had any sort of a problem with alcohol. This is the first I've heard of it."
In the long term
Alcohol has shown to have some long-term effect on working memory. Findings have shown that for working memory to be substantially affected, long-term, heavy drinking must be sustained over a long period of time as up to one drink per day does not impair cognitive function and may actually decrease the risk of cognitive decline.[57] Furthermore, chronic alcoholism is associated with impairment in sustained attention and visual working memory. Thus, alcoholics have reduced ability, but not necessarily inability, to perform these executive tasks, assumed to be subserved by regions of prefrontal cortex.[58] While it may not serve as a surprise that chronic alcoholism has been linked to decreased cognitive function including working memory, one surprising finding is not only that even moderate levels of alcohol consumption during pregnancy were shown to have an adverse effect on the child's working memory when tested at 7.5 years of age, but that working memory may be the most important aspect of attention that is adversely affected by prenatal alcohol exposure.[59]
Prospective memory
Prospective memory involves remembering to carry out an intended action in the future without an explicit reminder.[31] Alcohol has been found to impair this ability. Chronic heavy alcohol users report significantly more prospective forgetting compared to low-dose and alcohol-free controls.[60] The Prospective Memory Questionnaire assesses short-term habitual prospective memory, long-term episodic prospective memory, and internally cued prospective memory.[60] Chronic heavy alcohol users reported significantly greater deficits for all three aspects of prospective memory.[60] Individuals that report heavy alcohol use report 24% more difficulties with prospective memory than those who report that they are light drinkers and 30% more difficulties than those who report that they never drink.[61] The effects of alcohol on prospective memory can also be assessed in the laboratory by simulating prospective memory tasks that individuals face in everyday life. Individuals who are given 0.6 g/kg alcohol prior to performing prospective memory tasks do significantly poorer than a placebo group.[62] Alcohol can damage the prefrontal and frontal areas of the brain, and this may be responsible for prospective memory impairments since prospective memory performance is highly correlated with frontal executive functions.[60]
In popular culture
The memory inhibiting effects of alcohol are often a prominent topic in popular culture. It appears in movies, books, and television shows. Several movies show characters drinking alcohol to the point of memory loss and awakening the next morning with a host of problems due to actions they performed while intoxicated.
One example is The Hangover, where three groomsmen lose the groom during a bachelor party in Las Vegas, so they retrace their steps to find him.[63] The characters still had functioning implicit/procedural memory, which allowed them to carry out the many acts they performed that night, but their episodic memory was impaired and thus they had no recollection of the events occurring. In addition to alcohol the characters were also under the influence of flunitrazepam.
Another movie is What Happens in Vegas. After an intoxicated night in "Sin City," two people wake-up to find they got married.[64]
Songs such as Waking Up in Vegas by Katy Perry[65] and Last Name by Carrie Underwood[66] also depict characters waking up and not remembering the night before due to alcohol consumption.
By some accounts, popular culture makes light of the memory problems that can result from alcohol consumption.
The court case R. v. Daviault [1994] concerned the viability of a legal defense based on intoxication.
Law and Order: Special Victims Unit; in season 11 episode 4, Hammered, a recovering alcoholic was coersed to drink by a business partner and later wakes up to a dead woman in his bed.
See also
References
- ↑ Merriam-Webster's Online Dictionary
- ↑ Rose, M. E.; Grant, J. E. (2010). "Alcohol-Induced Blackout". Journal of Addiction Medicine. 4 (2): 61–73. PMID 21769024. doi:10.1097/ADM.0b013e3181e1299d.
- ↑ Bliss, T. V. P.; Collingridge, G. L. (1993). "A synaptic model of memory: Long-term potentiation in the hippocampus". Nature. 361 (6407): 31–39. PMID 8421494. doi:10.1038/361031a0.
- ↑ Zola-Morgan, S.; Squire, L. R.; Amaral, D. G. (1986). "Human amnesia and the medial temporal region: Enduring memory impairment following a bilateral lesion limited to field CA1 of the hippocampus". The Journal of neuroscience : the official journal of the Society for Neuroscience. 6 (10): 2950–2967. PMID 3760943.
- ↑ White, A. M.; Best, P. J. (2000). "Effects of ethanol on hippocampal place-cell and interneuron activity". Brain Research. 876 (1–2): 154–165. PMID 10973604. doi:10.1016/S0006-8993(00)02629-9.
- ↑ Ryabinin AE, Criado JR, Henriksen SJ, Bloom FE, Wilson MC (1997). Differential sensitivity of c-Fos expression in hippocampus and other brain regions to moderate and low doses of alcohol. Mol Psychiatry, (1),32-43.
- ↑ Ryabinin AE (1998). Role of hippocampus in alcohol-induced memory impairment: implications from behavioral and immediate early gene studies. Psychopharmacology, 139, 1-2.
- ↑ Weiner JL, Gu C, Dunwiddie TV (1997). Differential Ethanol Sensitivity of Subpopulations of GABAA Synapses Onto Rat Hippocampal CA1 Pyramidal Neurons. The Journal of Neurophysiology,77(3), 1306-1312
- ↑ White A (2003). What Happened? Alcohol, Memory Blackouts and the Brain. Alcohol Research & Health, 27(2),186-196
- ↑ Paul, S. (2006). Alcohol-sensitive GABA receptors and alcohol antagonists. Published online 22 May 2006. doi:10.1073/pnas.0602862103.
- 1 2 3 Kumar, S.; Porcu, P.; Werner, D. F.; Matthews, D. B.; Diaz-Granados, J. L.; Helfand, R. S.; Morrow, A. L. (2009). "The role of GABAA receptors in the acute and chronic effects of ethanol: A decade of progress". Psychopharmacology. 205 (4): 529–564. PMC 2814770
 . PMID 19455309. doi:10.1007/s00213-009-1562-z.
. PMID 19455309. doi:10.1007/s00213-009-1562-z. - ↑ Proctor, W. R.; Poelchen, W.; Bowers, B. J.; Wehner, J. M.; Messing, R. O.; Dunwiddie, T. V. (2003). "Ethanol Differentially Enhances Hippocampal GABAA Receptor-Mediated Responses in Protein Kinase Cgamma (PKCgamma ) and PKCepsilon Null Mice". Journal of Pharmacology and Experimental Therapeutics. 305 (1): 264–270. PMID 12649378. doi:10.1124/jpet.102.045450.
- ↑ Park, S. K.; Sedore, S. A.; Cronmiller, C.; Hirsh, J. (2000). "Type II cAMP-dependent Protein Kinase-deficient Drosophila Are Viable but Show Developmental, Circadian, and Drug Response Phenotypes". Journal of Biological Chemistry. 275 (27): 20588–20596. PMID 10781603. doi:10.1074/jbc.M002460200.
- ↑ Silvers, J. M.; Tokunaga, S.; Berry, R. B.; White, A. M.; Matthews, D. B. (2003). "Impairments in spatial learning and memory: Ethanol, allopregnanolone, and the hippocampus". Brain research. Brain research reviews. 43 (3): 275–284. PMID 14629930. doi:10.1016/j.brainresrev.2003.09.002.
- ↑ Lovinger DM, White G, Weight FF (1989). Ethanol inhibits NMDA-activated ion current in hippocampal neurons. Science, 243(4899), 1721-1724
- ↑ Lovinger, D. M.; White, G.; Weight, F. F. (1990). "NMDA receptor-mediated synaptic excitation selectively inhibited by ethanol in hippocampal slice from adult rat". The Journal of neuroscience : the official journal of the Society for Neuroscience. 10 (4): 1372–1379. PMID 2158533.
- ↑ Peoples, R. W.; Stewart, R. R. (2000). "Alcohols inhibit N-methyl-D-aspartate receptors via a site exposed to the extracellular environment". Neuropharmacology. 39 (10): 1681–1691. PMID 10884550. doi:10.1016/S0028-3908(00)00067-8.
- ↑ Morrisett, R. A.; Swartzwelder, H. S. (1993). "Attenuation of hippocampal long-term potentiation by ethanol: A patch-clamp analysis of glutamatergic and GABAergic mechanisms". The Journal of neuroscience : the official journal of the Society for Neuroscience. 13 (5): 2264–2272. PMID 8478698.
- ↑ Swartzwelder, H. S.; Wilson, W. A.; Tayyeb, M. I. (1995). "Age-dependent inhibition of long-term potentiation by ethanol in immature versus mature hippocampus". Alcoholism: Clinical and Experimental Research. 19 (6): 1480–1485. PMID 8749814. doi:10.1111/j.1530-0277.1995.tb01011.x.
- ↑ Pian, J. P.; Criado, J. R.; Milner, R.; Ehlers, C. L. (2010). "NMDAR subunit expression in adult and adolescent brain following chronic ethanol exposure". Neuroscience. 170 (2): 645–654. PMC 2945397
 . PMID 20603193. doi:10.1016/j.neuroscience.2010.06.065.
. PMID 20603193. doi:10.1016/j.neuroscience.2010.06.065. - ↑ Pascual, M.; Boix, J.; Felipo, V.; Guerri, C. (2009). "Repeated alcohol administration during adolescence causes changes in the mesolimbic dopaminergic and glutamatergic systems and promotes alcohol intake in the adult rat". Journal of Neurochemistry. 108 (4): 920–931. PMID 19077056. doi:10.1111/j.1471-4159.2008.05835.x.
- ↑ Lai, C. C.; Kuo, T. I.; Lin, H. H. (2007). "The Role of Protein Kinase a in Acute Ethanol-Induced Neurobehavioral Actions in Rats". Anesthesia & Analgesia. 105 (1): 89–96. PMID 17578962. doi:10.1213/01.ane.0000263030.13249.36.
- ↑ Schummers, J.; Browning, M. D. (2001). "Evidence for a role for GABA(A) and NMDA receptors in ethanol inhibition of long-term potentiation". Brain research. Molecular brain research. 94 (1–2): 9–14. PMID 11597760. doi:10.1016/s0169-328x(01)00161-9.
- ↑ Besheer, J.; Cox, A. A.; Hodge, C. W. (2003). "Coregulation of Ethanol Discrimination by the Nucleus Accumbens and Amygdala". Alcoholism: Clinical and Experimental Research. 27 (3): 450–456. PMID 12658110. doi:10.1097/01.ALC.0000057036.64169.C1.
- ↑ Besheer, J.; Hodge, C. W. (2004). "Pharmacological and Anatomical Evidence for an Interaction Between mGluR5- and GABAA α1-Containing Receptors in the Discriminative Stimulus Effects of Ethanol". Neuropsychopharmacology. 30 (4): 747–757. PMC 2892057
 . PMID 15549054. doi:10.1038/sj.npp.1300616.
. PMID 15549054. doi:10.1038/sj.npp.1300616. - ↑ Hodge, C. W.; Cox, A. A. (1998). "The discriminative stimulus effects of ethanol are mediated by NMDA and GABA(A) receptors in specific limbic brain regions". Psychopharmacology. 139 (1–2): 95–107. PMID 9768547. doi:10.1007/s002130050694.
- ↑ Ming Z, Criswell HE, Yu G, Breese GR (2006). Competing presynaptic and postsynaptic effects of ethanol on cerebellar purkinje neurons. Alcohol, Clinical and Experimental Research,30(8),1400-1407.
- ↑ Waltman C, Blevins Jr LS, Boyd G, Wand GS (1993). The effects of mild ethanol intoxication on the hypothalamic-pituitary-adrenal axis in nonalcoholic men. Journal of Clinical Endocrinology & Metabolism, 77, 518-522
- ↑ Halpern-Felsher BL, Millstein SG, Ellen JM (1996). Relationship of alcohol use and risky sexual behavior: a review and analysis of findings. Journal of Adolescent Health, 19(5),331–336.
- ↑ Sun MK, Reis DJ (1992). Effects of systemic ethanol on medullary vasomotor neurons and baroreflexes. Neuroscience Letters, 137(2),232-236.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 Baddeley, A., Eysenck, M.W. and Anderson, M.C. (2009). Memory. New York, NY: Psychology Press.
- 1 2 3 Soderlund, H., Parker, E.S., Schwartz, B.L., and Tulving, E. (2005). Memory encoding and retrieval on the ascending and descending limbs of the blood alcohol concentration curve. Psychopharmacology, 182, 305-317.
- 1 2 Lee, H., Roh S., & Kim, D. (2009). Alcohol-Induced Blackout. International Journal of Environmental Research and Public Health, 6, 2783-2792.
- 1 2 3 Parker, Birnbaum & Noble. (1976) Alcohol and Memory: Storage and State Dependency. Journal of Verbal Learning and Verbal Behaviour, 15, 691-702.
- 1 2 3 Nelson, T., McSpadden, M., Fromme, K., & Marlatt, G. (1986). Effects of Alcohol Intoxication on Metamemory and on Retrieval from Long-Term Memory. Journal of Experimental Psychology, 115(3), 247-254.
- 1 2 Haut, J., Beckwith, B., Petros, T., & Russell, S. (1989). Gender Differences in Retrieval From Long-Term Memory Following Acute Intoxication With Ethanol. Physiology and Behaviour, 45, 1161-1165.
- ↑ Miller, G.A. (1994). The magical number seven, plus or minus two: some limits on our capacity for processing information. Psychological Review, 101(2), 343-352.
- 1 2 3 4 5 Saults, J.S., Cowan, N., Sher, K.J., and Moreno, M.V. (2007). Differential short Term effects of Alcohol on Working Memory: Distinguishing Multiple Processes. Experimental and Clinical Psychopharmacology, 15(6), 576-587.
- ↑ Bates, M. E. (2006). Acute alcohol effects on repetition priming and word recognition memory with equivalent memory cues. Brain Cogn, 60, 118-127.
- ↑ Eun, L., Dong-Pyo, J., Jae-Jin, K., Suk Kyoon, A., Sangjin, P., In-Young, K., Sun I.K., Kang-Jun, Y., & Kee, N. (2007). Alteration of brain metabolites in young alcoholics without structural changes. NeuroReport, 18(14), 1511-1514.
- ↑ Tapert, S.F., Brown, G.G., Kindermann, S.S., Cheung, E.H., Frank, L.R., & Brown, S.A. (2006). fMRI measurement of brain dysfunctions in alcohol-dependent young women. Alcoholism: Clinical and Experimental Research, 25(2), 26-245.
- ↑ Warrington, E. K., James, M., £ Maciejewski, C. (1986). The WAIS as a lateralizing and localizing diagnostic instrument: A study of 656 patients with unilateral cerebral lesions. Neuropsychologia, 24, 223-239.
- ↑ Goldman-Rakic, P. S. (1992). Working memory and the mind. Scientific American, 267, 110-117.
- 1 2 3 Sullivan, E.V., Rosenbloom, M.J., Lim, K.O., & Pfefferbam, A. (2000). Longitudinal changes in cognition, gait, and balance in abstinent and relapsed alcoholic men: relationships to changes in brain structure. Neuropsychology, 14(2), 178-188.
- ↑ Subhan. (1983). The effects of midazolam in conjunction with alcohol in iconic memory and free recall. Neuropsychobiology, 9(4), 230-234.
- 1 2 3 4 Ray, S., & Bates, M.E. (2007). Acute effects of alcohol on intrusion errors in free recall tasks. International Journal on Disability and Human Development. Special issue: Alcohol related cognitive disorders, 6(2) 201-205.
- ↑ Hashtroudi, S., Parker, DeLisi, L.E., Wyatt, R.J., & Mutter, S.A. (1984). Intact retention in acute alcohol amnesia. Journal of Experimental Psychology: Learning, Memory, and Cognition, 10(1), 156-163.
- ↑ Tracy, J.I., & Bates, M.E. (1999). The selective effects of alcohol on automatic and effortful memory processes. Neuropsychology, 13(2), 282-290.
- ↑ Williams, H.L., & Rundell, U.H. (1984). Effect of alcohol on recall and recognition as functions of processing levels. Journal of Studies on Alcohol, 45(1), 10-15.
- ↑ Wegner, A.J., & Fahle, M. (1999). Alcohol and visual performance. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 23(3), 465-482.
- 1 2 Miller, M.E., Adesso, V.J., Fleming, J.P., Gino, A., & Lauerman, R. (1978). Journal of Experimental Psychology: Human Learning and Memory, 4(3), 246-255.
- ↑ Wegner, A.J., & Fahle, M. (1999). Alcohol and visual performance. Prog. neuro-psychopharmacol. & biol. psychiat, 23, 465-482.
- 1 2 Baddeley, A.(1998) Comptes Rendus de l'Academie des Sciences – serie III, 321(2-3), 167-173.
- ↑ Paulus, Martin P, Tapert, Susan F, Pulido, Carmen, & Schuckit, Marc A. (2006). Alcohol attenuates load-related activation during a working memory task: Relation to level of response to alcohol. Dept of Psychiatry, University of California. PP 2
- 1 2 3 Finn, P.R., Justus, A., Mazas, C., and Steinmetz, J.E. (1999). Working memory, executive processes and the effects of alcohol on Go/No-Go learning: testing a model of behavioral regulation and impulsivity. Psychopharmacology, 146, 465-472.
- ↑ Grattan-Miscio, K.E. and Vogel-Sprott, M. (2005) Effects of alcohol and performance incentives on immediate working memory. Springer Berlin / Heidelberg, 188-196
- ↑ Stampfer MJ, Kang JH, Chen J, Cherry R, Grodstein F (2005). "Effects of moderate alcohol consumption on cognitive function in women". N Engl J Med. 352: 245–253. PMID 15659724. doi:10.1056/NEJMoa041152.
- ↑ Pfefferbaum A, Desmond JE, Galloway C, Menon V, Glover GH, Sullivan EV (July 2001). "Reorganization of frontal systems used by alcoholics for spatial working memory: an fMRI study". NeuroImage. 14 (1 pt 1). PMID 11525339. doi:10.1006/nimg.2001.0785.
- ↑ Burden MJ, Jacobson SW, Sokol RJ, Jacobson JL (March 2005). "Effects of prenatal alcohol exposure on attention and working memory at 7.5 years of age". Alcohol Clin Exp Res. 29 (3): 443–52. PMID 15770121. doi:10.1097/01.ALC.0000156125.50577.EC.
- 1 2 3 4 Heffernan, T.M., Moss, M and Ling, J. (2002). Subjective Ratings of Prospective Memory Deficits in Chronic Heavy Alcohol Users. Alcohol & Alcoholism, 37(3), 269-271.
- ↑ Ling, J., Heffernan, T.M., Buchanan, T., Rodgers, J., Scholey, A.B., and Parrot, A.C. (2003). Effects of Alcohol on Subjective Ratings of Prospective and Everyday Memory Deficits. Alcoholism: Clinical and Experimental Research, 27(6), 970-974
- ↑ Leitz, J.R., Morgan, C. A. J., Bisby, J.A., Rendell, P.G., Curran, V.H. (2009). Global impairment of prospective memory following acute alcohol. Psychopharmacology, 205, 379-387.
- ↑ "The Hangover" (2009). Retrieved from http://www.imdb.com/title/tt1119646/.
- ↑ "What Happens in Vegas" (2008). Retrieved from http://www.imdb.com/title/tt1033643/plotsummary.
- ↑ "YouTube – Waking Up in Vegas Lyrics – Katy Perry" (2009). Retrieved from https://www.youtube.com/watch?v=06Qf7GdAF70
- ↑ "YouTube – Carrie Underwood – Last Name" (2009). Retrieved from https://www.youtube.com/watch?v=f27zNlmRMWU