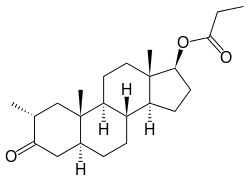
Drostanolone propionate
 | |
| Clinical data | |
|---|---|
| Trade names | Masteron, Drolban, Masteril, Mastisol, Metormon, Permastril |
| Pregnancy category |
|
| Routes of administration | Intramuscular injection or transdermal cream |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability |
Oral: 0–2% Intramuscular: 100% |
| Protein binding | Highly |
| Metabolism | Hepatic |
| Biological half-life | 2–3 Days |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| ChEBI | |
| ECHA InfoCard | 100.007.550 |
| Chemical and physical data | |
| Formula | C23H36O3 |
| Molar mass | 360.53 g/mol |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Drostanolone propionate (BAN) (brand names Masteron, Drolban, others), also known as dromostanolone propionate (USAN), as well as 2α-methyl-4,5α-dihydrotestosterone, is a synthetic anabolic-androgenic steroid (AAS) and the propionate ester of drostanolone.[1][2] It is incapable of aromatization and has similar properties to dihydrotestosterone (DHT). It has been successfully used to treat breast cancer, but because of the high risk of virilization, options with better tolerability are usually prescribed instead.[3] Drostanolone propionate is not orally active, and must be administered instead via intramuscular injection.
References
- ↑ J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 652–. ISBN 978-1-4757-2085-3.
- ↑ Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 377–. ISBN 978-3-88763-075-1.
- ↑ Chowdhury MS, Banks AJ, Bond WH, Jones WG, Ward HW (1976). "A comparison of drostanolone propionate (Masteril) and nandrolone decanoate (Deca-durabolin) in the treatment of breast carcinoma". Clin Oncol. 2 (3): 203–6. PMID 1036981.
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.