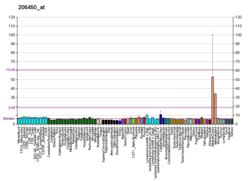
Dopamine beta-hydroxylase
Dopamine beta-hydroxylase (DBH), also known as dopamine beta-monooxygenase, is an enzyme (EC 1.14.17.1) that in humans is encoded by the DBH gene. Dopamine beta-hydroxylase catalyzes the chemical reaction:
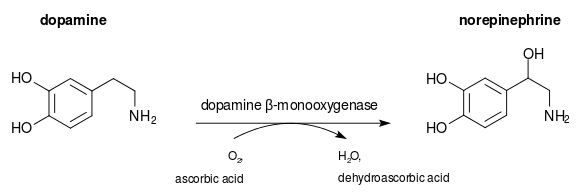
The three substrates of this enzyme are 3,4-dihydroxyphenethylamine, ascorbate, and O2, whereas its three products are noradrenaline, dehydroascorbate, and H2O.
DBH is a 290 kDa copper-containing oxygenase consisting of four identical subunits, and its activity requires ascorbate as a cofactor.[3]
It is the only enzyme involved in the synthesis of small-molecule neurotransmitters that is membrane-bound, making norepinephrine the only known transmitter synthesized inside vesicles. It is expressed in noradrenergic nerve terminals of the central and peripheral nervous systems, as well as in chromaffin cells of the adrenal medulla.
Mechanism of catalysis
| dopamine beta-monooxygenase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC number | 1.14.17.1 | ||||||||
| CAS number | 9013-38-1 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / EGO | ||||||||
| |||||||||
Based on the observations of what happens when there's no substrate, or oxygen, the following steps seem to constitute the hydroxylation reaction.[4][5]
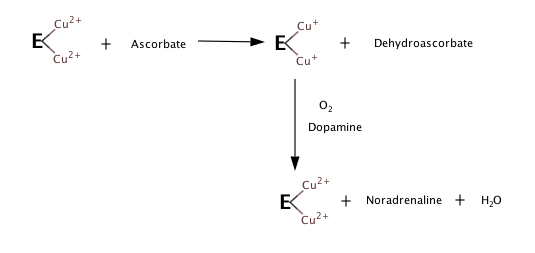
Although details of DBH mechanism are yet to be confirmed, DBH is homologous to another enzyme, peptidylglycine α-hydroxylating monooxygenase (PHM). Because DBH and PHM share similar structures, it is possible to model DBH mechanism based on what is known about PHM mechanism.[6]
Substrate specificity
Dopamine beta-hydroxylase catalyzes the hydroxylation of not only dopamine but also other phenylethylamine derivatives when available. The minimum requirement seems to be a benzene ring with a two-carbon side chain that terminates in an amino group.[4]
Clinical significance
DBH primarily contributes to catecholamine and trace amine biosynthesis. It also participates in the metabolism of xenobiotics related to these substances; for example, the human DBH enzyme catalyzes the beta-hydroxylation of amphetamine and para-hydroxyamphetamine, producing norephedrine and para-hydroxynorephedrine respectively.[10][11][12]
DBH has been implicated as correlating factor in conditions associated with decision making and addictive drugs, e.g., alcoholism[13] and smoking,[14] attention deficit hyperactivity disorder,[15] schizophrenia,[16] and Alzheimer's disease.[17] Inadequate DBH is called dopamine beta hydroxylase deficiency.
Structure
Because it is difficult to obtain a stable crystal of dopamine beta-hydroxylase, its crystal structure is yet to be solved. However, an homology model based on the primary sequence and comparison to PHM is available.[18]
Regulation and inhibition
This protein may use the morpheein model of allosteric regulation.[19]
Inhibitors
| HYD[lower-alpha 1] | HP[lower-alpha 2] | QCA[lower-alpha 3] | IQCA[lower-alpha 4] | BI[lower-alpha 5] | IAA[lower-alpha 6] | |
|---|---|---|---|---|---|---|
| Competitive | Ascorbate | Ascorbate | Ascorbate | Ascorbate | Ascorbate | Ascorbate |
| Uncompetitive | Tyramine | Tyramine | ||||
| Mixed | Tyramine | Tyramine | Tyramine | Tyramine | ||
| Ascorbate is cofactor; tyramine is substitute for dopamine, DBH's namesake substrate | ||||||
DBH is inhibited by disulfiram,[20] tropolone,[21] and, most selectively, by nepicastat.[22]
DBH is reversibly inhibited by l-2H-Phthalazine hydrazone (hydralazine; HYD), 2-1H-pyridinone hydrazone (2-hydrazinopyridine; HP), 2-quinoline-carboxylic acid (QCA), l-isoquinolinecarboxylic acid (IQCA), 2,2'-bi-lH-imidazole (2,2'-biimidazole; BI), and IH-imidazole-4-acetic acid (imidazole-4-acetic acid; IAA). HYD, QCA, and IAA are allosteric competitive.[23]
Nomenclature
The systematic name of this enzyme class is 3,4-dihydroxyphenethylamine, ascorbate:oxygen oxidoreductase (beta-hydroxylating).
Other names in common use include:
- dopamine beta-monooxygenase
- dopamine beta-hydroxylase
- membrane-associated dopamine beta-monooxygenase (MDBH)
- soluble dopamine beta-monooxygenase (SDBH)
- dopamine-B-hydroxylase
- 3,4-dihydroxyphenethylamine beta-oxidase
- 4-(2-aminoethyl) pyrocatechol beta-oxidase
- dopa beta-hydroxylase
- dopamine beta-oxidase
- dopamine hydroxylase
- phenylamine beta-hydroxylase
- (3,4-dihydroxyphenethylamine) beta-mono-oxygenase
References
- ↑ "Human PubMed Reference:".
- ↑ "Mouse PubMed Reference:".
- ↑ Rush RA, Geffen LB (1980). "Dopamine beta-hydroxylase in health and disease". Critical Reviews in Clinical Laboratory Sciences. 12 (3): 241–77. PMID 6998654. doi:10.3109/10408368009108731.
- 1 2 Kaufman S, Bridgers WF, Baron J (1968). "The Mechanism of Action of Dopamine beta-Hydroxylase.". Advances in Chemistry. 77, chapter 73: 172–176. doi:10.1021/ba-1968-0077.ch073.
- ↑ Friedman S, Kaufman S (May 1966). "An electron paramagnetic resonance study of 3,4-dihydroxyphenylethylamine beta-hydroxylase". The Journal of Biological Chemistry. 241 (10): 2256–9. PMID 4287853.
- ↑ Prigge ST, Mains RE, Eipper BA, Amzel LM (August 2000). "New insights into copper monooxygenases and peptide amidation: structure, mechanism and function". Cellular and Molecular Life Sciences. 57 (8–9): 1236–59. PMID 11028916. doi:10.1007/pl00000763.
- ↑ Broadley KJ (March 2010). "The vascular effects of trace amines and amphetamines". Pharmacol. Ther. 125 (3): 363–375. PMID 19948186. doi:10.1016/j.pharmthera.2009.11.005.
- ↑ Lindemann L, Hoener MC (May 2005). "A renaissance in trace amines inspired by a novel GPCR family". Trends Pharmacol. Sci. 26 (5): 274–281. PMID 15860375. doi:10.1016/j.tips.2005.03.007.
- ↑ Wang X, Li J, Dong G, Yue J (February 2014). "The endogenous substrates of brain CYP2D". Eur. J. Pharmacol. 724: 211–218. PMID 24374199. doi:10.1016/j.ejphar.2013.12.025.
The highest level of brain CYP2D activity was found in the substantia nigra ... The in vitro and in vivo studies have shown the contribution of the alternative CYP2D-mediated dopamine synthesis to the concentration of this neurotransmitter although the classic biosynthetic route to dopamine from tyrosine is active. ... Tyramine levels are especially high in the basal ganglia and limbic system, which are thought to be related to individual behavior and emotion (Yu et al., 2003c). ... Rat CYP2D isoforms (2D2/2D4/2D18) are less efficient than human CYP2D6 for the generation of dopamine from p-tyramine. The Km values of the CYP2D isoforms are as follows: CYP2D6 (87–121 μm) ≈ CYP2D2 ≈ CYP2D18 > CYP2D4 (256 μm) for m-tyramine and CYP2D4 (433 μm) > CYP2D2 ≈ CYP2D6 > CYP2D18 (688 μm) for p-tyramine
- ↑ Glennon RA (2013). "Phenylisopropylamine stimulants: amphetamine-related agents". In Lemke TL, Williams DA, Roche VF, Zito W. Foye's principles of medicinal chemistry (7th ed.). Philadelphia, USA: Wolters Kluwer Health/Lippincott Williams & Wilkins. pp. 646–648. ISBN 9781609133450. Retrieved 11 September 2015.
The phase 1 metabolism of amphetamine analogs is catalyzed by two systems: cytochrome P450 and flavin monooxygenase. ... Amphetamine can also undergo aromatic hydroxylation to p-hydroxyamphetamine. ... Subsequent oxidation at the benzylic position by DA β-hydroxylase affords p-hydroxynorephedrine. Alternatively, direct oxidation of amphetamine by DA β-hydroxylase can afford norephedrine.
- ↑ Taylor KB (January 1974). "Dopamine-beta-hydroxylase. Stereochemical course of the reaction" (PDF). J. Biol. Chem. 249 (2): 454–458. PMID 4809526. Retrieved 6 November 2014.
Dopamine-β-hydroxylase catalyzed the removal of the pro-R hydrogen atom and the production of 1-norephedrine, (2S,1R)-2-amino-1-hydroxyl-1-phenylpropane, from d-amphetamine.
- ↑ Horwitz D, Alexander RW, Lovenberg W, Keiser HR (May 1973). "Human serum dopamine-β-hydroxylase. Relationship to hypertension and sympathetic activity". Circ. Res. 32 (5): 594–599. PMID 4713201. doi:10.1161/01.RES.32.5.594.
Subjects with exceptionally low levels of serum dopamine-β-hydroxylase activity showed normal cardiovascular function and normal β-hydroxylation of an administered synthetic substrate, hydroxyamphetamine.
- ↑ Mutschler J, Abbruzzese E, Witt SH, Dirican G, Nieratschker V, Frank J, Grosshans M, Rietschel M, Kiefer F (August 2012). "Functional polymorphism of the dopamine β-hydroxylase gene is associated with increased risk of disulfiram-induced adverse effects in alcohol-dependent patients". Journal of Clinical Psychopharmacology. 32 (4): 578–80. PMID 22760354. doi:10.1097/jcp.0b013e31825ddbe6.
- ↑ Ella E, Sato N, Nishizawa D, Kageyama S, Yamada H, Kurabe N, Ishino K, Tao H, Tanioka F, Nozawa A, Renyin C, Shinmura K, Ikeda K, Sugimura H (June 2012). "Association between dopamine beta hydroxylase rs5320 polymorphism and smoking behaviour in elderly Japanese". Journal of Human Genetics. 57 (6): 385–90. PMID 22513716. doi:10.1038/jhg.2012.40.
- ↑ Bhaduri N, Sinha S, Chattopadhyay A, Gangopadhyay PK, Singh M, Mukhopadhyay KK (February 2005). "Analysis of polymorphisms in the dopamine beta hydroxylase gene: association with attention deficit hyperactivity disorder in Indian children". Indian Pediatrics. 42 (2): 123–9. PMID 15767706.
- ↑ Cubells JF, Sun X, Li W, Bonsall RW, McGrath JA, Avramopoulos D, Lasseter VK, Wolyniec PS, Tang YL, Mercer K, Pulver AE, Elston RC (November 2011). "Linkage analysis of plasma dopamine β-hydroxylase activity in families of patients with schizophrenia". Human Genetics. 130 (5): 635–43. PMC 3193571
 . PMID 21509519. doi:10.1007/s00439-011-0989-6.
. PMID 21509519. doi:10.1007/s00439-011-0989-6. - ↑ Combarros O, Warden DR, Hammond N, Cortina-Borja M, Belbin O, Lehmann MG, Wilcock GK, Brown K, Kehoe PG, Barber R, Coto E, Alvarez V, Deloukas P, Gwilliam R, Heun R, Kölsch H, Mateo I, Oulhaj A, Arias-Vásquez A, Schuur M, Aulchenko YS, Ikram MA, Breteler MM, van Duijn CM, Morgan K, Smith AD, Lehmann DJ (2010). "The dopamine β-hydroxylase -1021C/T polymorphism is associated with the risk of Alzheimer's disease in the Epistasis Project". BMC Medical Genetics. 11 (161): 162. PMC 2994840
 . PMID 21070631. doi:10.1186/1471-2350-11-162.
. PMID 21070631. doi:10.1186/1471-2350-11-162. - 1 2 Kapoor A, Shandilya M, Kundu S (2011). "Structural insight of dopamine β-hydroxylase, a drug target for complex traits, and functional significance of exonic single nucleotide polymorphisms". PLOS ONE. 6 (10): e26509. PMC 3197665
 . PMID 22028891. doi:10.1371/journal.pone.0026509.
. PMID 22028891. doi:10.1371/journal.pone.0026509. - ↑ Selwood T, Jaffe EK (March 2012). "Dynamic dissociating homo-oligomers and the control of protein function". Archives of Biochemistry and Biophysics. 519 (2): 131–43. PMC 3298769
 . PMID 22182754. doi:10.1016/j.abb.2011.11.020.
. PMID 22182754. doi:10.1016/j.abb.2011.11.020. - ↑ Goldstein M, Anagnoste B, Lauber E, Mckeregham MR (July 1964). "INHIBITION OF DOPAMINE-BETA-HYDROXYLASE BY DISULFIRAM". Life Sciences. 3 (7): 763–7. PMID 14203977. doi:10.1016/0024-3205(64)90031-1.
- ↑ Goldstein M, Lauber E, Mckereghan MR (July 1964). "THE INHIBITION OF DOPAMINE-BETA-HYDROXYLASE BY TROPOLONE AND OTHER CHELATING AGENTS". Biochemical Pharmacology. 13 (7): 1103–6. PMID 14201135. doi:10.1016/0006-2952(64)90109-1.
- ↑ Stanley WC, Li B, Bonhaus DW, Johnson LG, Lee K, Porter S, Walker K, Martinez G, Eglen RM, Whiting RL, Hegde SS (August 1997). "Catecholamine modulatory effects of nepicastat (RS-25560-197), a novel, potent and selective inhibitor of dopamine-beta-hydroxylase". British Journal of Pharmacology. 121 (8): 1803–9. PMC 1564872
 . PMID 9283721. doi:10.1038/sj.bjp.0701315.
. PMID 9283721. doi:10.1038/sj.bjp.0701315. - ↑ Townes S, Titone C, Rosenberg RC (February 1990). "Inhibition of dopamine beta-hydroxylase by bidentate chelating agents". Biochimica et Biophysica Acta. 1037 (2): 240–7. PMID 2306475. doi:10.1016/0167-4838(90)90174-E.
Further reading
- Friedman S, Kaufman S (December 1965). "3,4-dihydroxyphenylethylamine beta-hydroxylase. Physical properties, copper content, and role of copper in the catalytic acttivity". The Journal of Biological Chemistry. 240 (12): 4763–73. PMID 5846992.
- Levin EY, Levenberg B, Kaufman S (1960). "The enzymatic conversion of 3,4-dihydroxyphenylethylamine to norepinephrine". J. Biol. Chem. 235: 2080–2086.
External links
- GeneReviews/NIH/NCBI/UW entry on Dopamine Beta-Hydroxylase Deficiency
- Dopamine beta-Hydroxylase at the US National Library of Medicine Medical Subject Headings (MeSH)