Domoic acid
 | |
| Names | |
|---|---|
| IUPAC name
(2S,3S,4S)-3-(Carboxymethyl)-4-[(1Z,3E,5R)-6-hydroxy-1,5-dimethyl-6-oxo-hexa-1,3-dienyl]pyrrolidine-2-carboxylic acid | |
| Identifiers | |
| 3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.159.099 |
| PubChem CID |
|
| |
| |
| Properties | |
| C15H21NO6 | |
| Molar mass | 311.33 g·mol−1 |
| Density | 1.273 g/cm3 |
| Vapor pressure | 2.62×10−16 mmHg (34.9 fPa) |
| Hazards | |
| R-phrases (outdated) | R20 R21 R22 |
| S-phrases (outdated) | S36 S37 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Domoic acid (DA) is a kainic acid analog neurotoxin that causes amnesic shellfish poisoning (ASP).[1] It is produced by algae and accumulates in shellfish, sardines, and anchovies. When sea lions, otters, cetaceans, humans etc., then eat contaminated animals, poisoning may result. Exposure to this compound affects the brain, causing seizures, and possibly death.[2]
History
There has been little use of domoic acid throughout history except for in Japan, where it has been used as an anthelmintic for centuries. Domoic acid was first isolated in 1959 from a species of red algae, Chondria armata, in Japan; commonly referred to as "doumoi" (in Tokunoshima's dialect word) or "hanayanagi". Poisonings in history have been rare, or undocumented; however, it is thought that the increase in human activities is resulting in an increasing frequency of toxic algal blooms along coastlines in recent years. Consequently, poisonings have been affecting sea animals, birds, and humans.[3]
In 1961, seabirds attacked the Capitola area in California, and though it was never confirmed, they were thought to be under the influence of domoic acid.[4]
In 1987, on Prince Edward Island, Canada, there was a shellfish poisoning resulting in 3 deaths. Blue mussels (Mytulis edulis) contaminated with domoic acid were blamed.[5]
An incident in which domoic acid may have been involved took place on June 22, 2006, when a California brown pelican flew through the windshield of a car on the Pacific Coast Highway.[6]
Chemistry
General
Domoic acid is a structural analog of kainic acid, proline, and endogenous excitatory neurotransmiter glutamate.[8] Ohfune and Tomita, who wanted to investigate its absolute stereochemistry, were the first and only to synthesize domoic acid in 1982.[9]
Biosynthesis
In 1999, using 13C- and 14C-labelled precursors, the biosynthesis of domoic acid in the diatom genus Pseudo-nitzschia was examined. After addition of [1,2-13C2]-acetate, NMR spectroscopy showed enrichment of every carbon in domoic acid, indicating incorporation of the carbon isotopes. This enrichment was consistent with two biosynthetic pathways. The labeling pattern determined that domoic acid can be biosynthesized by an isoprenoid intermediate in combination with a tricarboxylic acid (TCA) cycle intermediate.[10]
Synthesis
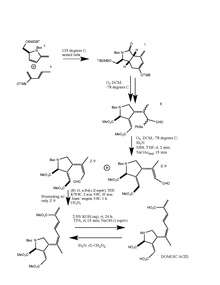
Using intermediates 5 and 6, a Diels-Alder reaction produced a bicyclic compound (7). 7 then underwent ozonolysis to open the six-membered ring leading to selenide (8). 8 was then deselenated to form 9 (E-9 and Z-9), lastly leading to the formation of (-) domoic acid.[11]
Mechanism of action
The effects of domoic acid have been attributed to several mechanisms, but the one of concern is through glutamate receptors. Domoic acid is an excitatory amino acid analogue of glutamate; a neurotransmitter in the brain that activates glutamate receptors. Domoic acid has a very strong affinity for these receptors, which results in excitotoxicity initiated by an integrative action on ionotropic glutamate receptors at both sides of the synapse blocking the channel from rapid desensitization. In addition there is a synergistic effect with endogenous glutamate and N-Methyl-D-aspartate receptor agonists that contribute to the excitotoxicity.
In the brain, domoic acid especially damages the hippocampus and amygdaloid nucleus. It damages the neurons by activating AMPA and kainate receptors, causing an influx of calcium. Although calcium flowing into cells is normal, the uncontrolled increase of calcium causes the cells to degenerate. Because the hippocampus may be severely damaged, short-term memory loss occurs. It may also cause kidney damage – even at levels considered safe for human consumption, a new study in mice has revealed. The kidney is affected at a hundred times lower than the concentration allowed under FDA regulations.[12][13]
Symptoms
| Humans | Other Animals |
|---|---|
| vomiting | head weaving |
| nausea | seizures |
| diarrhea and abdominal cramps within 24 hours of ingestion | bulging eyes |
| headache | mucus from the mouth |
| dizziness | disorientation |
| confusion, disorientation | death |
| loss of short-term memory | |
| motor weakness | |
| seizures | |
| profuse respiratory secretions | |
| cardiac arrhythmias | |
| coma and possible death |
Toxicology
Domoic acid producing algal blooms are associated with the phenomenon of amnesic shellfish poisoning (ASP). Domoic acid can bioaccumulate in marine organisms such as shellfish, anchovies, and sardines that feed on the phytoplankton known to produce this toxin. It can accumulate in high concentrations in the tissues of these plankton feeders when the toxic phytoplankton are high in concentration in the surrounding waters. Domoic acid is a neurotoxin that inhibits neurochemical processes, causing short-term memory loss, brain damage, and, in severe cases, death in humans. In marine mammals, domoic acid typically causes seizures and tremors.
Studies have shown that there are no symptomatic effects in humans at levels of 0.5 mg/kg of body weight. In the 1987 domoic acid poisoning on Prince Edward Island concentrations ranging from 0.31–1.28 mg/kg of muscle tissue were noted in people that became ill (three of whom died). Dangerous levels of domoic acid have been calculated based on cases such as the one on Prince Edward island. The exact LD50 for humans is unknown; for mice the LD50 is 3.6 mg/kg.[15]
New research has found that domoic acid is a heat-resistant and very stable toxin, which can damage kidneys at concentrations that are 100 times lower than what causes neurological effects.[16]
Diagnosis and prevention
In order to be diagnosed and treated if poisoned, domoic acid must first be detected. Methods such as ELISA or probe development with polymerase chain reaction (PCR) may be used to detect the toxin or the organism producing this toxin.[17]
There is no known antidote available for domoic acid. Therefore, if poisoning occurs, it is advised to go quickly to a hospital. Cooking or freezing affected fish or shellfish tissue that are contaminated with domoic acid does not lessen the toxicity.[18]
As a public health concern, the concentration of domoic acid in shellfish and shellfish parts at point of sale should not exceed the current permissible limit of 20 mg/kg tissue. In addition during processing shellfish, it is important to pay attention to environmental condition factors.[19]
Pop culture
On August 18, 1961, in Capitola and Santa Cruz, California there was an invasion of what people described as chaotic seabirds. These birds were believed to be under the influence of domoic acid, and it inspired a scene in Alfred Hitchcock's feature film The Birds.[20]
In addition domoic acid was used to poison a witness in the Elementary episode "The Red Team".
Domoic acid overtakes Camp Kikiwaka causing the campers to develop bizarrely altered personalities in the TV Series Bunk'd Season 2 Episode 10.
See also
References
- ↑ Clayden, Jonathan; Read, Benjamin; Hebditch, Katherine. "Chemistry of domoic acid, isodomoic acids, and their analogues" (PDF). Retrieved 2 April 2015.
- ↑ "Domoic Acid Toxicity". The Marine Mammal Center. Retrieved 2 April 2015.
- ↑ "Domoic Acid Poisoning". Northwest Fisheries science center. Retrieved 17 April 2015.
- ↑ "DOMOIC ACID - A major concern to washington state’s shellfish lovers". Washington Department of Fish and wildlife. Retrieved 12 April 2015.
- ↑ Gilbert, Steven. "Domoic Acid". Toxipedia. Retrieved 15 April 2015.
- ↑ "Possibly drunk pelican hits windshield". NBC news. Retrieved 12 April 2015.
- ↑ Clayden, Jonathan; Read, Benjamin; Hebditch, Katherine. "Chemistry of domoic acid, isodomoic acids, and their analogues" (PDF). Retrieved 2 April 2015.
- ↑ http://onlinelibrary.wiley.com/doi/10.1002/ana.410370125/full
- ↑ Clayden, Jonathan; Read, Benjamin; Hebditch, Katherine. "Chemistry of domoic acid, isodomoic acids, and their analogues" (PDF). Retrieved 2 April 2015.
- ↑ Ramsey; Douglas; Walter; Wright (1999). "Biosynthesis of domoic acid by the diatom Pseudo-nitzschia multiseries". Natural Toxins. 6 (3–4): 137–46. PMID 10223629. doi:10.1002/(sici)1522-7189(199805/08)6:3/4<137::aid-nt28>3.0.co;2-l.
- ↑ Clayden, Jonathan; Read, Benjamin; Hebditch, Katherine. "Chemistry of domoic acid, isodomoic acids, and their analogues" (PDF). Retrieved 2 April 2015.
- ↑ Pulido, Olga (2008). "Domoic Acid Toxicologic Pathology: A Review". Mar Drugs. 6 (2): 180–219. PMC 2525487
 . PMID 18728725. doi:10.3390/md20080010.
. PMID 18728725. doi:10.3390/md20080010. - ↑ Smith, Torrey. "Toxin in seafood causes kidney damage in mice at levels considered safe for consumption". avante medical center.
- ↑ Gilbert, Steven. "Domoic Acid". Toxipedia. Retrieved 2 April 2015.
- ↑ Gilbert, Steven. "Domoic Acid". Toxipedia.
- ↑ Smith, Torrey. "Toxin in seafood causes kidney damage in mice at levels considered safe for consumption". avante medical center.
- ↑ "Detection and Analysis of Marine Biotoxins". Northwest fisheries science center.
- ↑ "Domoic Acid - A Major Concern to Washington State’s Shellfish Lovers". washington department of fish and wildlife.
- ↑ Kumar, K. Prem; Kumar, Sreeletha Prem; Nair, G. Achuthan (2009). "Risk assessment of the amnesic shellfish poison, domoic acid, on animals and humans". Journal of Environmental Biology. 30 (3): 319–25. PMID 20120452.
- ↑ Bargu, Sibel; Silver, Mary W.; Ohman, Mark D.; Benitez-Nelson, Claudia R.; Garrison, David L. (2011). "Mystery behind Hitchcock's birds". Nature Geoscience. 5 (1): 2–3. Bibcode:2012NatGe...5....2B. doi:10.1038/ngeo1360.
External links
- "Domoic Acid and Pseudo-nitzschia References". Fisheries and Oceans Canada. Archived from the original on 2013-12-04.
- "Amnesic Shellfish Poisoning, Domoic Acid, and Pseudo-nitzschia". International Society for the Study of Harmful Algae ISSHA.
- "Domoic acid". IPCS INCHEM.
- "Domoic Acid - A Major Concern to Washington State’s Shellfish Lovers". Washington Department of Fish and Wildlife.
- "Domoic Acid Toxicity".